Significant Figures and Scientific Notation for Chem Tech and Chem

Significan Digits
aka
Significant Figures
What are they?
They show the certainty (precision) of your measuring device.
0.1g
0.1000g
What’s the difference in these two numbers?
*Sig. Figs. are only for measurements. So always ask, is the value you are looking at a measurement of some kind. The sig. figs. for exact values are ignored.
*The last digit is always the digit that is least certain (the digit you are estimating).
More Difficult Rules
(1) All nonzero digits are significant:
1.234 g has 4 significant figures,
1.2 g has 2 significant figures.
(2) Zeroes between nonzero digits are significant:
1002 kg has 4 significant figures,
3.07 mL has 3 significant figures.
(3) Leading zeros to the left of the first nonzero digits are not significant; such zeroes merely indicate the position of the decimal point:
0.001
o
C has only 1 significant figure,
0.012 g has 2 significant figures.
(4) Trailing zeroes that are also to the right of a decimal point in a number are significant:
0.0230 mL has 3 significant figures,
0.20 g has 2 significant figures.
(5) When a number ends in zeroes that are not to the right of a decimal point, the zeroes are not necessarily significant:
190 miles may be 2 or 3 significant figures,
50,600 calories may be 3, 4, or 5 significant figures.
The potential ambiguity in the last rule can be avoided by the use of standard exponential, or "scientific," notation. For example, depending on whether the number of significant figures is 3, 4, or 5, we would write 50,600 calories as:
5.06 × 10
5.060
4
× 10
4 calories (3 significant figures)
5.0600
× 10 4 calories (4 significant figures), or calories (5 significant figures).
Another potential ambiguity comes from an ending decimal
50,600. calories (5 significant figures)
The decimal makes all digits significant
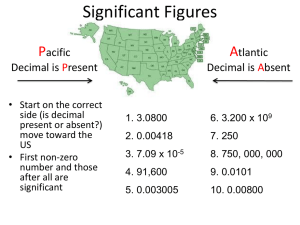
How do you find how many sig. figs. a number has?
If a decimal point is P resent, ignore zeros on the P acific (left) side (except if there are zeros between non-zero numbers; in this case all are significant from the first nonzero number on). If the decimal point is A bsent, ignore zeros on the A tlantic
(right) side. Everything else is significant.
Ex: 1) 0.00
3200 (4 sig. figs)
2) 1.003200 (7 sig. figs)
Doing math with Sig. figs.!
Adding and subtracting: line the numbers’ decimal points up. Add (or subtract) the numbers.
• To decide where to round, the result should have as many decimal places as the measured number with the smallest number of decimal places.
– Ex: 121.23 (two decimal places) + 1.2540 (four decimal places) = 122.4840
*Answer should round based on two decimal places which would give 122.48
Example #1
A) 13.559 + 1.22599 + 20.23=
B) 100.9552 – 29.059 ‒ 79.8 =
C) 0.98 – 0.099 – 0.422 =
Rounding to the right number of sig. figs.
Given a value, start from the left (using the concept of pacific ocean for decimal numbers) and begin counting the number of sig. figs. you are told to have.
Replace whole numbers with zeros for place holders, decimal numbers just leave them off.
Example #2
Round each number to three significant digits:
1. 0.0050505
2. 123040
3. 3500
4. 2.0309
5. 1.4592
How to multiply and divide?
Multiply or divide the numbers given.
Count the number of sig.figs. in each value.
Round your answer to the number of sig. figs. that has the least of the values.
So… if you are multiplying or dividing two values one with 3 sig. figs. and one with 2 sig. figs., your answer should only have two sig. figs.
Example #3
A) 25 x 2.39 x 0.1
B) 950/0.0359
C) 7.228 x 40.3
3.4
D) 6.53 x 0.00042
Add/subtract with multiply/divide
Break into components (like operations) and perform an order of operations. Follow individual rules for each component.
PEMDAS (Parenthesis, Exponent, Multiplication,
Division, Addition, Subtraction)
1. Parenthesis
2. Multiply/Division
3. Addition/Subtraction
Ex: 3.2 + 2.35 x 1.560 vs. (3.2 + 2.35) x 1.560
= 6.9
= 8.7
Example #4
A) (0.0035 – 0.0021)
0.0245
B) 375 + 27.33 x 25
C) 4.523
(2.2 – 2.1)
D) 0.036 – 3.2/1.37
Scientific Notation
It’s the way to handle very large or small numbers.
Ex: 0.0000000000032 is a very small number
Ex: 1,231,500,000 is a very large number
To go from a number to scientific notation
1) Find the first non-zero value and place a decimal in between it and the next number (located to the right).
2) Now, starting from your newly added decimal, count how many spaces are between the decimal you added and the original decimal. Each space represents a factor of 10 (why you see x 10 x ). If there is no decimal count from your newly added decimal to the end of number.
3) If you move left, your exponent (superscript) x will be negative the number of spaces counted. If you move right, your exponent (superscript) x will be positive the number of spaces counted.
4) Keep number of sig. figs. In original value.
Scientific Notation
It’s the way to handle very large or small numbers.
Ex: 0.0000000000032 is a very small number
1) 0.000000000003.2
2) There are 12 spaces to the left
3) Left tells you x 10 -12 and negative
4) There were two sig. figs. to begin with so converted value is now
3.2 x 10 -12
Ex: 1,231,500,000 is a very large number
1) 1.231500000
2) There are 9 spaces to the right (end of number)
3) Right tells you x 10 9 and positive (we do not put +)
4) There were 5 sig. figs. to begin with so converted value is now
1.2315 x 10 9
* You can also work backwards from scientific notation to number. If the exponent (superscript) is positive move that many spaces right and fill in zeros. If exponent (superscript) is negative, move that many spaces left and fill in zeros (keep decimal).
Example #5
A) 0.00068 (give scientific notation)
B) 1.38 x 10 5 (give number)
C) 585,000 (give scientific notation)
D) 6 x 10 -6 (give number)
E) 695.1 x 10 -4 (give standard scientific notation; tricky)
Example #6
Sig. Figs with scientific notation. How many sig. figs. does each number have?
A) 6.80 x 10 -3
B) 1.3821 x 10 5
C) 3 x 10 8
Round each to two sig. figs.
Sig. Figs. and Actual Measurements
• The number you estimate determines the sig. figs.
You can tell this is between 2.8 and 2.9. What numbers come between 2.8 and 2.9?
2.81, 2.82, 2.83, etc
*2.811 comes between 2.8 and 2.9 as well but in this case you would be estimating the last two decimal places.
-In this case the hundredths place determines the sig. figs. because it is the number you are estimating.
- Rule of thumb when taking a measurement, the number of sig. figs. will be a tenths place to the right of the decimal place you can actually read with a tick mark.