Notes: Significant Figures (Stop @ slide #11)
advertisement

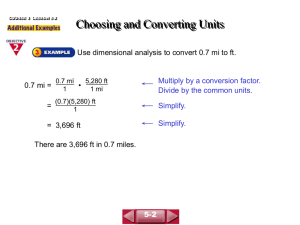
Performing these conversions is a lot like going on a road trip. Scientific Notation Scientist use special notation to express very large or very small numbers. Example I: 300,000,000 g can be written as… 3 x 108 g Ex II: 1,007,000,000 sec can be written as… 1.007 x 109 sec Ex III: 0.000 000 000 004 76 mL can be written as… 4.76 x 10-12 mL Significant Figures Atlantic - Pacific Rule: Decimal Present: Count from the Pacific side Decimal Absent: Count from the Atlantic side Start counting at the first non-zero number and count until you reach the end of the number Ex. I: 3.00700 Decimal Present… Pacific (left)… 6 sig. figs. Ex. II: 300,700 Decimal Absent… Atlantic (right)… 4 sig. figs. Before we take this trip, let’s review significant figures… Determine how many significant figures are in each of the following measurements. 1) 2) 3) 4) 5) 6) 0.0034050 L 33.600 m 7500.0 g 47,900 mm 7,000,000,001 miles 8.07 Hz 5 ___________ 5 ___________ ___________ 5 3 ___________ 10 ___________ ___________ 3 More practice… Round the following measurements off so that they each contain 3 significant figures. 7) 366.2 L 8) 9,047,022 mg 9) 12.76 g 10) 999.9 J ___________ 366 L 9,050,000 mg ___________ ___________ 12.8 g 1.00 x 103 J ___________ Notice this one must be in scientific notation to have 3 sig. figs. Significant Figures in Calculations When multiplying and dividing, limit and round to the least number of significant figures in any of the factors. Example: 23.0 x 432 x 19 = 188,784 = 190,000 The answer is expressed as 190,000 or 1.9 x 105 since 19 has only two sig. figs. Significant Figures in Calculations When adding and subtracting, limit and round your answer to the least number of decimal places in any of the numbers involved in the calculation. Example: 123.25 + 46.0 +86.257 = 255.507 = 255.5 The answer is expressed as 255.5 since 46.0 has only one decimal place. Before we take this trip, let’s review significant figures… Perform the prescribed operations. Round your answers to the proper # of sig. figs. 11.4 m/s 11)36.57 m / 3.21 s = ___________ 97.6 g 12)41.376g + 13.3g + 42.9g=___________ 76.2 m2 13)5.67 m x 13.44 m ___________ 1.0 m/s2 14)(5.83 m/ 2.67 s) /2.1 s ___________ 60 V 15)9.374 V x 6 ___________ From now on, we will round all our answers to the correct # of significant figures. Now back to our road trip… Let’s say you want to travel from Oracle Jct to Casa Grande... You must travel through Tucson… a two part trip. Rule: You must stay on the roads Rule: You need a map. Sometimes the map can be in your head. But, not at first. OK…Lets talk about making maps and solving conversion problems. The method we use is called the “Factor-Label” method, a.k.a. dimensional analysis. Given 1 kildurkin = 18 gallons For Example: gallons kildurkins This is our map. For Example: Given 1 kildurkin = 18 gallons gallons kildurkins How many kildurkins is 14 gallons? 14 gallons 1 kildurkins 18 gallons =0.78 kildurkins General Format... given going to coming from Let’s expand the map... Also given: 2 farkins = 1 kildurkin gallons kildurkins farkins Also given: 1 hogshead = 63 gallons 1 barrel = 3.3 bushels hogshead 1 bushel = 8 gallons gallons bushel barrel kildurkins farkins Now, Lets solve a problem. How many hogsheads are 14 barrels? Finish Here The trip will require 3 hogshead transitions 3 gallons kildurkins farkins 2 bushel 1 barrel Given: Start Here How many hogsheads are 14 barrels? hogshead Given 3 gallons 14 barrels 3.3 bushels 8 gallons 1 barrel 1 bushel 1 hogshead 63 gallons = 5.9 hogsheads 2 bushel 1 going to going to going to coming from coming from coming from barrel Now you try one. How many farkins is 3.00 bushels? End Here hogshead gallons kildurkins farkins Start Here bushel barrel This will require 3 Conversions. bushels gallons farkins kildurkins 3.00 bushels 8 gallons 1 kildurkin 2 farkins 1 bushel 1 kildurkin 18 gallons = 2.67 farkins Wow! How Cool Is That!