Tutorial: The Sun`s Nuclear Reactions
advertisement

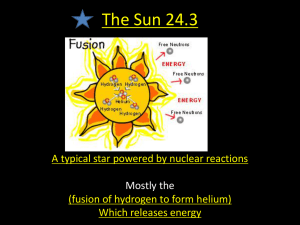
Sun Nuclear Reactions Sun Nuclear Physics The work of Einstein – A new view of mass: E = m c2 Conceptual Implication: Mass is a highly concentrated form of energy. Therefore mass can be converted to other forms of energy, as long as Conservation of Energy is satisfied Sun Nuclear Physics How much mass would be required to operate a 200 Megawatt power plant for one year? Note: The total amount of energy required to operate the facility for a year is 6.4 x 1015 Joules. Solution: m c2 = 6.4 x 1015 Joules 6.4 x 1015 m= (3 x 108)2 6.4 x 1015 = = .071 kg = 71 g 9x 1016 Note: the mass of a paperclip is about 1 g Sun Nuclear Physics – The Sun’s Core The nuclear reaction that produces the energy in the core of the sun is 4 ( 1H ) 4He + energy + 2 neutrinos Given the following information: Mass of 1H = 1.6736 x 10-27 Kg Mass of 4He = 6.6466 x 10-27 Kg What is the mass on the left side of this reaction? Total MassLeft Side = 4 times the mass of 1H = (4) (1.6736 x 10-27 kg) = 6.6943 x 10-27 kg Sun Nuclear Physics – The Sun’s Core The nuclear reaction that produces the energy in the core of the sun is 4 ( 1H ) 4He + energy + 2 neutrinos Given the following information: Mass of 1H = 1.6736 x 10-27 Kg Mass of 4He = 6.6466 x 10-27 Kg What is the mass on the right side of this reaction? Since there is no mass associated with the energy or the neutrinos on the right side, Total MassRight Side = 6.6466 x 10-27 kg Sun Nuclear Physics – The Sun’s Core The nuclear reaction that produces the energy in the core of the sun is 4 ( 1H ) 4He + energy + 2 neutrinos Given the following information: Mass of 1H = 1.6736 x 10-27 Kg Mass of 4He = 6.6466 x 10-27 Kg What is the difference in mass in this reaction? m = 4.77 x 10-29 kg Sun Nuclear Physics – The Sun’s Core The nuclear reaction that produces the energy in the core of the sun is 4 ( 1H ) 4He + energy + 2 neutrinos Given the following information: Mass of 1H = 1.6736 x 10-27 Kg Mass of 4He = 6.6466 x 10-27 Kg How much energy is released in this reaction? Energy = m c2 = 4.3 x 10-12 Joules per reaction Sun Nuclear Physics – The Sun’s Core How many nuclear reactions per second are required to account for the luminosity of the sun? Recall that he luminosity of the sun is 4 x 1026 J/sec. To determine the number of reactions per second, we must know the total energy released per second by the sun, and divide by the amount of energy per reaction, that is, Number of reactions per second = total energy per second/energy per reaction Therefore, Reactions per second = (4 x 1026 J/sec) / 4.3 x 10-12 Joules/reaction = 9.3 x 1037 reactions per second Sun Nuclear Physics – The Sun’s Core A kilogram of mass is equivalent to how much every (assume a perfect conversion of mass into energy)? Another way to think about it – how many joules of energy are contained in 1 kg of mass? Recall E = m c2 Therefore E/m = c2 = (3 x 108 m/sec)2 = 9 x 1016 J/kg Sun Nuclear Physics – The Sun’s Core A The units in the calculation of the previous slide should be m2 / sec2 . How did they magically become J/kg? The fundamental MKS units of the Joule is Joule → kg m2 / sec2 If you don’t remember this, think about he MKS units on Kinetic Energy It should be clear, therefore, that Joule / kg → m2 / sec2 And that the units on the previous slide are correct Sun Nuclear Physics – The Sun’s Core Given the luminosity of the sun, how many kilograms of mass are being converted to energy in 1 second? The mass converted per second can be determine by knowing the J/sec produced by the sun (luminosity) and dividing that number the by the J/kg calculated in the previous calculation. Therefore Mass / second = (Luminosity of the Sun) / (Energy – Mass Equivalent) = (4 x 1026 J/sec) / (9 x 1016 J/Kg) = 4.4 x 109 kg/sec !!! On Earth, this mass is conversion is equivalent to converting over 4 millions tons of material PER SECOND. Sun Nuclear Physics – The Sun’s Core How many hydrogen nuclei are converted into Helium every second in the core of the sun? Our discussions in class show that 4 hydrogen nuclei participate in the creation of each helium nucleus. As a result, the number of hydrogen being converted each second can be determine by multiplying the number of reactions per second (see previous slide) by 4, since 4 hydrogen nuclei participate in each reaction. Therefore # hydrogen nuclei converted into Helium every second is: ( 9.3 x 1037 reactions/second ) / ( 4 Hydrogen/reaction ) = 3.7 x 1038 Hydrogen/second Sun Nuclear Physics – The Sun’s Core How many kilograms of hydrogen are being converted to helium each second in the core of the sun? The mass of hydrogen being converted is determined by multiplying the number of hydrogen being converted each second and multiplying by the mass of each hydrogen nucleus. Since the mass of hydrogen is 1.67 x 10-27 kg, The mass of hydrogen being converted into Helium every second is ( 3.7 x 1038 Hydrogen/second ) (1.67 x 10-27 Kg/Hydrogen) = 6.18 x 1011 Kg/second That is, about 6 x 1011 Kg of hydrogen is being converted to Helium every second in the core of the sun. Sun Nuclear Physics – The Sun’s Core About 6 x 1011 Kg of hydrogen is being converted to Helium every second in the core of the sun. On the earth, this would be equivalent to Over 600,000 million tons of Hydrogen every second




![The Politics of Protest [week 3]](http://s2.studylib.net/store/data/005229111_1-9491ac8e8d24cc184a2c9020ba192c97-300x300.png)

