Stoichiometry: Calculations with Chemical Formulas and Equations
advertisement

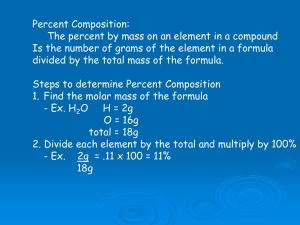
Unit 10: Stoichiometry 1 Calculations with Chemical Formulas Stoichiometry © 2009, Prentice-Hall, Inc. Mass in Elements and Compounds Stoichiometry © 2009, Prentice-Hall, Inc. Atomic Mass Atoms are so small, it is difficult to discuss how much they weigh in grams. • Use atomic mass units or amus – 1 amu is 1/12 the mass of a carbon-12 atom. This gives us a basis for comparison. • The decimal numbers on the periodic table are atomic masses in amu. Stoichiometry © 2009, Prentice-Hall, Inc. Gram Atomic Mass • Atomic mass in grams instead of amu’s. – Represents an amount that we can actually measure in lab. • Also known as a mole, or molar mass, which we will come back to later… Stoichiometry © 2009, Prentice-Hall, Inc. Gram Formula Mass • The gram formula mass is the sum of the atomic masses for the atoms in a chemical formula. • So, the gram formula mass of calcium chloride, CaCl2, would be Ca: 1 x 40.1 = 40.1 + Cl: 2 x 35.5 = 71.0 111.1 amu • Gram formula mass is generally used for either molecular or ionic compounds. Stoichiometry © 2009, Prentice-Hall, Inc. Gram Formula Mass • For the molecule, ethane (C2H6), the formula mass would be: C: 2 x 12.0 = 24.0 + H: 6 x 1.0 = 6.0 30.0 amu • For the ionic compound, (NH4)2CO3 N: 2 x 14.0 amu = 28.0 H: 8 x 1.0 amu = 8.0 C: 1 x 12.0 amu = 12.0 +O: 3 x 16.0 amu = 48.0 Stoichiometry = 96.0 amu © 2009, Prentice-Hall, Inc. Percent Composition You can find the percentage (%) of the mass of a compound that comes from each of the elements in the compound by using these steps: 1. Calculate the formula mass of the compound. 2. Divide the mass of each element by the formula mass and multiply that fraction x 100. Stoichiometry © 2009, Prentice-Hall, Inc. Percent Composition So the percentage of carbon in ethane,C2H6, is… C is 2 x 12.0 = 24.0 H is 6 x 1.0 = 6.0 30.0 % Carbon = 24.0 amu 30.0 amu = 80.0% x 100 amu Stoichiometry © 2009, Prentice-Hall, Inc. (stop) Stoichiometry © 2009, Prentice-Hall, Inc. Moles Stoichiometry © 2009, Prentice-Hall, Inc. Avogadro’s Number • 6.02 x 1023 • 1 mole of 12C has a mass of 12 g. Stoichiometry © 2009, Prentice-Hall, Inc. Molar Mass • By definition, a molar mass is the mass of 1 mol of a substance (i.e., g/mol). – The molar mass of an element is the mass number for the element that we find on the periodic table. – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol). Stoichiometry © 2009, Prentice-Hall, Inc. Using Moles Moles provide a bridge from the molecular scale to the real-world scale. Stoichiometry © 2009, Prentice-Hall, Inc. Mole Relationships • One mole of atoms, ions, or molecules contains Avogadro’s number of those particles. • One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound. Stoichiometry © 2009, Prentice-Hall, Inc. Finding Empirical Formulas Stoichiometry © 2009, Prentice-Hall, Inc. Calculating Empirical Formulas • Empirical formula: smallest, wholenumber ratio of atoms of elements in a compound. • You can calculate the empirical formula from the percent composition. Stoichiometry © 2009, Prentice-Hall, Inc. Steps for Empirical Formula 1. For each element, convert mass to moles. – If you have percents, use the percent as the number of grams/100 grams of compound. Example: 67% would be 67 grams. 2. Find the mole ratio: Divide all the numbers by the smallest number of moles 3. Use the smallest, whole number ratio as the subscripts for the formula. Stoichiometry © 2009, Prentice-Hall, Inc. Calculating Empirical Formulas Example: The compound para-aminobenzoic acid (you may have seen it listed as PABA on your bottle of sunscreen) is composed of: carbon (61.31%) hydrogen (5.14%) nitrogen (10.21%) oxygen (23.33%) Find the empirical formula of PABA. Stoichiometry © 2009, Prentice-Hall, Inc. Calculating Empirical Formulas Assuming 100.00 g of para-aminobenzoic acid, C: Sunscreen PABA carbon (61.31%) hydrogen (5.14%) nitrogen (10.21%) oxygen (23.33%) H: 1 mol 61.31 g x = 5.105 mol C 12.01 g 5.14 g x 1 mol = 5.09 mol H 1.01 g N: 1 mol 10.21 g x 14.01 g = 0.7288 mol N O: 1 mol 23.33 g x 16.00 g = 1.456 mol O Stoichiometry © 2009, Prentice-Hall, Inc. Calculating Empirical Formulas Calculate the mole ratio by dividing by the smallest number of moles: C: 5.105 mol 0.7288 mol = 7.005 = 7 H: 5.09 mol 0.7288 mol = 6.984 = 7 N: 0.7288 mol 0.7288 mol = 1.000 = 1 O: 1.458 mol 0.7288 mol = 2.001 = 2 Stoichiometry © 2009, Prentice-Hall, Inc. Calculating Empirical Formulas These numbers are the subscripts for the empirical formula: C7H7NO2 The molecule is shown here. Stoichiometry © 2009, Prentice-Hall, Inc. Let’s work some examples in your notes packet… Stoichiometry © 2009, Prentice-Hall, Inc. Molecular Formula Empirical Formula is the smallest whole number ratio of atoms of elements in a compound. Molecular Formula is the real formula of a compound. It is a multiple of the Empirical Formula. They may be the same! 1. Calculate the mass of the empirical formula. 2. Divide the molecular mass given in the problem by the empirical formula mass. 3. Multiply the subscripts in the empirical formula by the number you get to make new subscripts. Molecular formula is a multiple of the empirical formula! Stoichiometry © 2009, Prentice-Hall, Inc. Molecular Formula Problem • Analysis of a chemical used in photographic developing fluid indicates a chemical composition of: 65.4% C 5.45% H 29.09% O • The molar mass is found to be 110.0 g/mol. Determine the empirical and Stoichiometry molecular formulas. © 2009, Prentice-Hall, Inc. Find Empirical Formula First! 65.4g C 1 mole C = 5.45 ÷ 1.82 = 3 12g C 5.45g H 1 mole H = 5.45 ÷1.82 = 3 1g H 29.09g O 1 mole O = 1.82 ÷ 1.82 = 1 16g O C3H3O = Empirical Formula Stoichiometry © 2009, Prentice-Hall, Inc. Molecular Formula 1. Find molar mass C3H3O C – 3 x 12 = 36 H–3x1= 3 O – 1 x 16 = +16 55 g/mole is Empirical Formula Mass 2. Divide the given Molecular Formula Mass (110g/mole) by the Empirical Formula Mass 110/55 = 2, so C3x2H3x2O1x2 3. Molecular Formula = C6H6O2 Stoichiometry © 2009, Prentice-Hall, Inc. Molecular formula is some multiple of the empirical formula. It may be the same as the empirical formula! Two samples of a compound must have the same percent composition to be the same compound or they would have Stoichiometry different empirical formulas. © 2009, Prentice-Hall, Inc. Hydrates A hydrate is an ionic compound that has a specific number of water molecules bound to the atoms in its crystals. (This is NOT the same as being dissolved in water) Many compounds are found in nature as hydrates, such as protein crystals Stoichiometry © 2009, Prentice-Hall, Inc. Naming Hydrates To name a hydrate, give the name of the compound, and add the prefix for the number of waters + hydrate: CuSO4·5H2O copper(II) sulfate pentahydrate Stoichiometry © 2009, Prentice-Hall, Inc. Formula of a Hydrate 1. Weigh the hydrate, then heat it in a partly covered crucible to drive off the water. 2. Weigh it again to determine the amount of water lost from the anhydrous (no water) compound. Repeat until mass stops changing. 3. Calculate the empirical formula with the anhydrous (no water) compound as one unit and the water as the second. Stoichiometry © 2009, Prentice-Hall, Inc. Hydrate Problem Problem: Molar mass of CoCl2 If 11.75 g of cobalt(II) Co – 1 x 59 = 59 chloride is heated, 9.25 g Cl – 2 x 35.5 = +71 of anhydrous cobalt chloride, CoCl2, remains. 130 g/mol What is the formula and name for this hydrate? 9.25 g CoCl2 1 mole = .0712 ÷.0712 = 1 130 g 11.75 – 9.25 = 2.5 g water removed 9.25 g anhydrous CoCl2 2.5 g H20 1 mole = .139 ÷ .0712 = 2 18 g CoCl2·2H2O is cobalt(II) chloride dihydrate Stoichiometry © 2009, Prentice-Hall, Inc. Hydrate Problem A hydrate is found to have the following percent composition: 48.8% MgSO4 and 51.22% H2O. What is the formula and name of this hydrate? Molar mass of MgSO4 = 24.3 + 32 + 64 =120.3g/mol Molar mass of H2O = 2 + 16 = 18 g/mol = .406 ÷ .406 = 1 51.22 g 1 mole = 2.85 ÷ .406 = 7 48.8g 1 mole 120.3 g 18 g Stoichiometry Magnesium sulfate heptahydrate: MgSO4· 7H 2O © 2009, Prentice-Hall, Inc. Hydrates Sometimes hydration can have nifty color changes! Stoichiometry © 2009, Prentice-Hall, Inc. (end) Stoichiometry © 2009, Prentice-Hall, Inc. Empirical Formula • A blue solid is found to contain 36.84% nitrogen and 63.16% oxygen. What is the empirical formula for this solid? • Determine the empirical formula for a compound that contains 35.98% aluminum and 64.02% sulfur. Stoichiometry © 2009, Prentice-Hall, Inc.