Lecture 38
advertisement

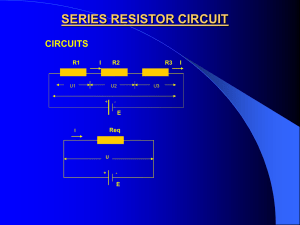
Review for Final Exam Schedule Review Session on Monday at 4:00 PM in 101 UPL if it extends beyond 6:00 PM it will move to 707 Keen. Final exam – Tuesday, April 23 at 7:30 AM in 101 Carroway building. This Power Point presentation contains 36 slides – most likely I won’t get to more than about 1/3 of them. Make sure you understand the concepts and problems presented here. B-Field Due to Currents Electric currents produce magnetism. B = oI/(2r) (due to a long straight wire) Direction from the right-hand rule. B I B I Curl your fingers as if following B. Your thumb is in the direction of the current. Example: Mass Spectrometer. F = qvB F = ma q,v R ma = qvB a = qvB/m qvB/m = v2/R R = mv/(qB) B is out of the page. Example: Mass Spectrometer. F = qvB q,v F = ma = mv2/R = qvB mv = qBR or R p = qBR ½mv2 = (mv)2/(2m) = p2/(2m) Energy = ½mv2 = ½(qBR)2/m B is out of the page. q < 0 !! Forces Between Currents I1 F12 B2 F21 I2 F12/L = I1B2 = I1oI2/(2r) = o I1 I2/(2r) B2 I1 F12 B2 Forces Between Currents B1 I1 F12 B1 F21 I2 F21 I2 B1 Major Concepts Battery (voltages) Ohm’s Law: V = IR Resistance: R = V/I (in ohms “”) Resistivity: R = L/A ( in /m) Power: P = I2R (resistors) Power: P = VI (batteries or resitors) Major Concepts Series Circuit Current must go through all resistors R T = R 1 + R2 + R 3 + … Parallel Circuit Current is divided between the resistors 1/RT = 1/R1 + 1/R2 + 1/R3 + … Terminal Voltage Accounts for the resistance within the battery. Vterminal = V – Ir(internal) Current in a Simple Circuit A V=8V + - R = 24 C Arrow shows the direction that positive charges move. V = IR I = V/R I = 8 V/24 B I = 0.33 Amp Current at A = 0.33 Amps Current at B = 0.33 Amps Current at C = 0.33 Amps Current is the same everywhere in the circuit! Energy in a Simple Circuit B V=8V + - A R = 24 D Recall: I = 1/3 Amp Consider the energy of a proton moving through the circuit. (recall: q = +1 e) Energy(A) = qVA = 8 eV C Proton loses energy moving from B to C. It gains energy moving from D to A. Energy(B) = qVB = 8 eV Energy(C) = qVC – q(IR) = 8eV – 1e * ( (1/3)*24 V ) = 0 eV Energy(D) = qVD = 0 eV Energy in a Simple Circuit B V=8V + - A R = 24 D C An electron loses energy moving from C to B. It gains energy moving from A to D. Recall: I = 1/3 Amp Consider the energy of a proton moving through the circuit. (recall: q = -1 e) Energy(D) = qVA = -8 eV Energy(C) = qVB = -8 eV Energy(B) = qVC – q(IR) = -8eV – -1e*((1/3)*24 V ) = 0 eV Energy(A) = qVD = 0 eV Circuit Analysis Kirchhoff’s Rules: Loop Rule: The sum of the voltage drops around any closed loop is zero. Conservation of Energy V = 0 Junction Rule: The net current into and out of any point in a circuit is zero. Charge conservation. I = 0 Complex Circuits R1 A V I1 C F R2 H B G I2 I3 E R1 = 10 , R2 = 3 , R3 = 6 and V = 24 Volts R3 D 1. I1 goes from A to B and from E to H. 2. I2 goes from B to F to G to E. 3. I3 goes from B to C to D to E. Complex Circuits R1 A V I1 C F R2 H B I2 I3 1. Junction Rule at B: I1 - I2 - I3 = 0 R3 2. Loop Rule: ABFGEHA -I1R1 – I2R2 + V = 0 G E D 3. Loop Rule: ABCDEHA -I1R1 – I3R3 + V = 0 Complex Circuits R1 A V I1 C F R2 H B G I2 I3 I1 - I2 - I3 = 0 -I1R1 – I2R2 + V = 0 R3 So: -10I1 – 3I2 + 24 = 0 -I1R1 – I3R3 + V = 0 E D So: -10I1 – 6I3 + 24 = 0 Solving the equations. I1 - I2 - I3 = 0 10I1 + 3I2 - 24 = 0 10I1 + 6I3 - 24 = 0 10I1 + 3I2 - 24 = 0 - ( 10I1 + 6I3 - 24 = 0 ) 3I2 – 6I3 = 0 3I2 = 6I3 I2 = 2I3 Solving the equations. I1 - I2 - I3 = 0 I2 = 2I3 I1 - 2I3 - I3 = 0 I1 - 3I3 = 0 I1 = 3I3 10I1 + 6I3 - 24 = 0 10*3I3 + 6I3 - 24 = 0 OR: 36I3 - 24 = 0 I3 = 24/36 = 0.667 A I1 = 3I3 I1 = 3* 0.667 A = 2.0 A Solving the equations. I1 - I2 - I3 = 0 I2 = 2I3 I3 = 24/36 = 2/3 A I1 = 3I3 I1 = 3* 2/3 A = 2 A I2 = 2I3 = 2 * 2/3 = 4/3 A Check the Equations I1 = 2 A, I2 = 4/3 A, I3 = 2/3 A I1 - I 2 - I3 = 0 10I1 + 3I2 - 24 = 0 2 – 4/3 – 2/3 = 0 10*2 + 3*4/3 – 24 = 0 10I1 + 6I3 - 24 = 0 10*2 + 6*2/3 – 24 = 0 Balancing the Energy P(in) = VI1 = 24 * 2 = 48 W P1 = I12R1 = 22*10 = 40 W P2 = I22R2 = (4/3)2*3 = 16/3 W P3 = I32R3 = (2/3)2*6 = 8/3 W PT = 40 + 16/3 + 8/3 = 48 W Reflected Light The angle of incidence equals the angle of reflection. in out Refracted Light air The direction changes when the light moves from material to another. glass The change depends on the material. in Snell’s Law determines the angles: out N1 sin 1 = N2 sin 2 The index of refraction, N, is the ratio of the speed of light in vacuum to the speed of light in the material. N = c/v c = speed of light in vacuum. v = speed of light in material. Lenses 1 2 object 1 – Parallel Ray 2 – Central Ray 3 – Focal Ray 3 image Optic Axis Lens Equation 1 1 1 — = — + — F O I F image object O I Optic Axis Diverging Lens 1 3 object Optic Axis image 1 – Parallel Ray 2 – Central Ray 3 – Focal Ray 2 Diverging Lens 1 3 object Optic Axis image F = - 20 cm O = 50 cm Use the Lens Equation 2 Diverging Lens w/ Lens Equation 1 1 1 — = — + — F O I F = - 20 cm O = 50 cm 1/(-20) = 1/50 + 1/I 1/I = -1/20 – 1/50 = -5/100 – 2/100 1/I = -7/100 I = -100/7 = - 14.28 cm m = -I/O = - (-14.28 cm)/( 50 cm ) = + 2/7 m > 0 Image is upright. I < 0 image is virtual. Atomic Model Electrons moved around nucleus only in certain stable orbits. Stable orbits are those in which an integral number of wavelengths fit into the diameter of the orbit (2rn = n) They emitted (absorbed) light only when they changed from one orbital to another. Orbits have quanta of angular momenta. L = nh/2 Orbit radius increases with energy rn = n2 r1 (r1 = .529 x 10-10 m) Atomic Energy Levels En = Z2/n2 Ionized atom E1 n=3 Hydrogen En = - 13.6 eV/n2 - 3.4 eV E = - 13.6 eV n=2 n=1 Emission & Absorption Energy is conserved. E = Eatom = Ef - Ei Photon energy = hf = hc/ Absorption photon disappears a electron in the atom changes from a lower energy level to a higher energy level. Emission an electron in atom goes from higher energy level to a lower energy level. This change in energy is the energy of the photon. Heisenberg Uncertainty Principle Impossible to know both the position and the momentum of a particle precisely. A restriction (or measurement) of one, affects the other. x p h/(2) Similar constraints apply to energy and time. E t h/(2) EXAMPLE: If an electron's position can be measured to an accuracy of 1.96×10-8 m, how accurately can its momentum be known? x p h/(2) p = h/(2x) p = 6.63x10-34 Js /(2 1.96x10-8 m) = 5.38 x 10-27 N s Nuclear Decay Rates N = -No t Number of decaying nuclei (in a given time t), depends on: Number of remaining nuclei, No Nuclear decay constant for that type of nuclei, Number of nuclei remaining after a time t: N = No e-t Nuclear Decay Rates Nuclear Decay Nuclei Remaining 1000.0 At t = N is 1/e (0.368) of the original amount 800.0 600.0 400.0 200.0 0.0 0.0 1.0 2.0 3.0 Time(s) 4.0 5.0 Example 10 5 B ( 01 n , 24 He ) 37 Li 1 0 n + 10 5 B 10 7 3 Li + 4 2 He B ( n, )7 Li Start with 5 protons end up with 5 protons. Start wit 11 baryons end up with 11 baryons. Q-value: (Mass Energy of Final State – Mass Energy of Initial State) Mn = 1.008665 u + M(B) = 10.012936 u = 11.021601 u M = 4.002602 u + M(L) = 7.016003 u = 11.018605 u Q = M (in MeV) = Mf – Mi = 11.018605 u - 11.021601 u Q = -0.002996 u = -0.002996 u * 931.5 MeV/u = -2.79 MeV Negative Q-value means we get extra energy out of the reaction. Example: Nuclear Power 1. Suppose that the average power consumption, day and night, in a typical house is 340 W. What initial mass of 235U would have to undergo fission to supply the electrical needs of such a house for a year? (Assume 200 MeV is released per fission.) Energy used = rate * time = 340 W * 3.15 x 107 s/yr = 1.07 x 1010 J Energy from each nucleus is: 200 MeV/nuclei = 200 MeV /nuclei * 1.60 x 10-13J/MeV = 3.2 x 10-11 J/nuclei Number of nuclei required = Energy/(Energy per nucleus) Number of nuclei = 1.07 x 1010 J/3.2 x 10-11 J/nuclei = 3.34 x 1020 nuclei Total mass of 235U = number of nuclei * mass/nucleus Mass = 3.34 x 1020 nuclei * 235 amu * 1.66 x 10-27 kg/amu = 1.31 10-4 kg