InorgCh15.1
advertisement
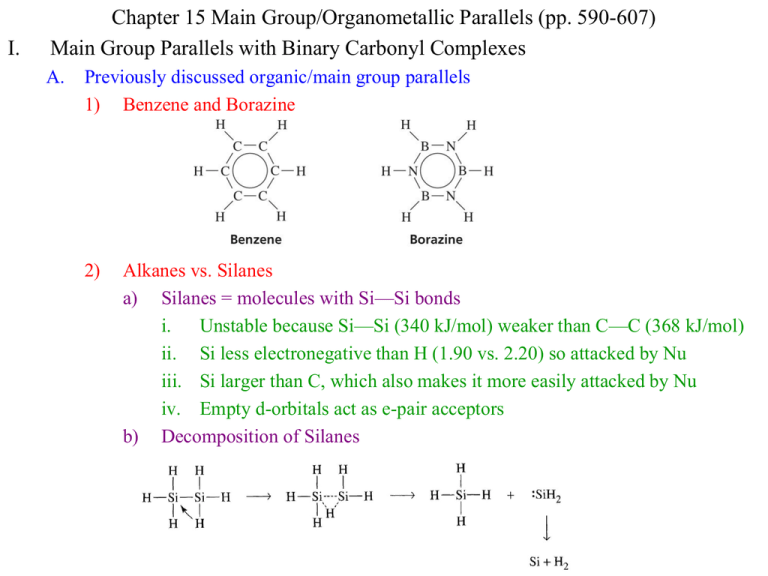
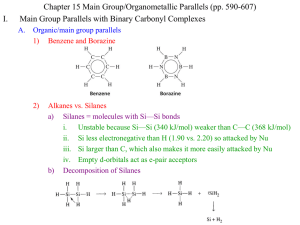
I. Chapter 15 Main Group/Organometallic Parallels (pp. 590-607) Main Group Parallels with Binary Carbonyl Complexes A. Previously discussed organic/main group parallels 1) Benzene and Borazine 2) Alkanes vs. Silanes a) Silanes = molecules with Si—Si bonds i. Unstable because Si—Si (340 kJ/mol) weaker than C—C (368 kJ/mol) ii. Si less electronegative than H (1.90 vs. 2.20) so attacked by Nu iii. Si larger than C, which also makes it more easily attacked by Nu iv. Empty d-orbitals act as e-pair acceptors b) Decomposition of Silanes B. Parallels between Main Group and Binary Carbonyl Organometallic Species 1) Electron count in relation to filled valence shells 2) Chemistry can be explained by species achieving filled valence shells 3) Group 5 Elements and 15 Electron Complexes: P vs. Ir(CO)3 4) II. Limitations of parallels a) No organometallic analogue for expanded valence shell of S, P, Cl, etc… b) No analogues of IF7 or XeF4 c) Many organometallic complexes with ligands weaker than CO don’t follow the 18 electron rule, thus parallels to the main group fail d) Loss of CO is much easier than loss of outer atoms from main group element The Isolobal Analogy A. Molecular Fragments are called “Isolobal” if the number, symmetry, energy, and shape of the frontier orbitals and the number of electrons in them are similar 1) Roald Hoffman, Nobel Prize 1982 2) Metal complexes can have similar reactivity/properties to main group molecules or fragments with which they are isolobal: Methane fragments vs Oh complex B. Molecular Fragments can be combined in to molecules, even between groups 1) All of the compounds below are known and stable 2) These parallels aren’t always so perfect a) H2C=CH2 is, of course, stable, but (CO)4Fe=F(CO)4 is not b) Yet, both fragments form stable 3-membered rings, (but with extra CO’s) C. Extending the Analogy 1) Charged species can be included a) Mn(CO)5 CH3 [Fe(CO)5]+ [Cr(CO)5]b) Coordination number should be the same 2) Gain or loss of electrons from two isolobal fragments yields isolobal fragments a) CH3 [Fe(CO)5]+ [Cr(CO)5]- (7e- or 17e- species) b) CH3+ [Fe(CO)5]2+ [Cr(CO)5]o (6e- or 16e- species) c) CH3- [Fe(CO)5]o [Cr(CO)5]2- (8e- or 18e- species) 3) Other ligands besides CO can be used a) Mn(CO)5 CH3 Mn(PR3)5 [Mn(Cl)5]5b) Cp and Benzene ligands are taken to occupy 3 sites and be 6e- doners c) Mn(CO)5 CH3 Mn(Cp)(CO)2 Mn(C6H6)(CO)2 4) Octahedral MLn fragments are isolobal with Square-Planar MLn-2 fragments Pt2+, d8, 14eLobe = LUMO Pto, d10, 16eLobes = non-bonding Cro, d6, 16eLobe = LUMO Feo, d8, 18eLobes = non-bonding D. Applications of the Isolobal Analogy 1) Seek new molecules made from isolobal fragments 2) P analogue of Cp a) 5e- CH unit of Cp is isolobal to P atom b) Combine 5 P atoms just like 5 CH units to produce a P5 ring like Cp 3) Linear Au versions of Mn(CO)5 a) Can combine these fragments similarly with CH3, each other, etc… III. Metal-Metal Multiple Bonds (p. 601-607) A. Parallel to Organic Bonds 1) Organic bonding: s, p orbitals give 1 s, and 2 p bonds 2) Metals: s, p, d orbitals give 1 s, 2 p, and 2 d B. Discovery 1) 1935 discovery of very close metal ions, closer than in solid, pure metal (240 vs. 275 pm) 2) Cotton discovers M-M bonds in 1963 crystal structures C. Bonding 1) Quadruple bonding is routine in M-M interactions 2) d-orbital interactions with other d-orbitals are most important for M-M bonding 3) Bonding in [Re2Cl8]2a) Re3+, d4 gives 8 d eb) Ligand e- go into new bonding MO c) 8 d-electrons go into s, p, p, d d) Result is BO = 4 e) d bond is week, but prevents rotation f) d-d* distance matches visible light [Re2Cl8]2- is blue [Mo2Cl8]4- is red g) Multiply bonded main group elements are colorless (N2, CO) because p-p* distance is UV h) [Os2Cl8]2- has Os3+ d5, 10 d ed* populated, BO = 3, staggered Cl8 group orbitals Matching dx2-y2 symmetry 1.dx2-y2 used for bonding to ligand 2.This modifies the available M-M MO’s Re2Cl82- d4 x 2 = 8 d e-, BO = 4 (d bond, eclipsed) Os2Cl82- d5 x 2 = 10 d e-, BO = 3 (no d bond, staggered) Cl8 group orbital interacts only with dx2-y2 group 4) Additional examples 5) Formal Shortness Ratio = multiple bond distance/single bond distance a) Shows the effect of multiple bonds on bond distance 6) Quintuple Bonds a) Cr-Cr and Mo-Mo complexes with quintuple (maximum) bonding b) Cr-Cr Bond length of 174.0pm and formal shortening ratio of 0.733 smallest reported