First Law for open systems (Lecture 3)
advertisement

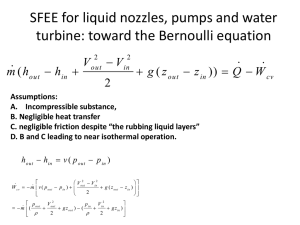
First Law of Thermodynamics FOR OPEN SYSTEMS Inserting expression for flow work W flow pv m outlets pv m inlets and regrouping terms dE C V m (u pv v / 2 gz ) 2 inlets m ( u pv v / 2 gz ) Q W C V 2 outlets For rate processes dividing both sides by t and letting t0 dE C V dt m ( h V / 2 gz ) 2 inlets m (h V 2 Recall enthalpy defn.: h=u+pv / 2 g z ) Q net , in W C V ou tl e ts For large processes provided all inlet/outlet conditions are steady (not changing with time) integrate both sides ECV inlets m ( h V / 2 gz ) 2 out lets m ( h V / 2 gz ) Q net , in W C V 2 The steady flow process • • • A process during which the fluid flows steadily through the control volume (CV) – Flow process fluid flows through CV. – Steady not changing with time During a steady flow process: – conditions (fluid properties, flow velocity, elevation) at any fixed point within the CV are unchanging with time. – Properties, flow velocity or elevation may change from point to point within CV – Size, shape, mass and energy content of the CV do not change with time. – Rate at which heat and work interactions take place with surroundings do not change with time. Devices/systems which undergo steady flow process: compressors, pumps, turbines, water supply pipes, nozzles, heat exchangers, power plants, aircraft engines etc. Conservation of mass and energy for a steady flow process dm C V dt dE C V dt . Q WCV m m (h V m (h V 2 gz ) 2 m (h V out m 2 gz ) 2 Conservation of mass inlets 2 2 m outlets in outlets outlets m inlet s . gz ) Q W C V inlets m (h V 2 gz ) 2 Conservation of energy Conservation of mass for the steady flow process • Mass balance for steady flow inlets m m outlets • Assuming uniform 1D flow at each inlet/outlet m AV v • A single stream (single inlet-single outlet) control volume m1 m 2 1 2 A1V1 v1 A2V 2 v2 Applications of SFEE • Nozzles and diffusers (e.g. jet propulsion) • Turbines (e.g. power plant, turbofan/turbojet aircraft engine), compressors and pumps (power plant) • Heat exchangers (e.g. boilers and condensers in power plants, evaporator and condenser in refrigeration, food and chemical processing) • Mixing chambers (power plants) • Throttling devices (e.g. refrigeration, steam quality measurement in power plants) All elements of a simple power plant/ refrigeration cycle and more! In principle, you can take the elements together to calculate power generated/required, heat removed/supplied. Applications of SFEE in pictures Heat exchangers Source: internet Throttling devices SFEE applied to nozzles/diffusers m (h V 2 2 outlets . gz ) Q W C V inlets Single stream hout V out 2 2 hin V in 2 2 m (h V 2 2 gz ) SFEE applied to turbines m ( hout hin V 2 out 2 Usually ) Q W cv V out V in 2 hout hin V 2 in 2 2 m ( hout hin ) Q W c v m ( hi n hou t ) 0 Enthalpy changes and kinetic energy: typical magnitudes • For example, saturated steam at 100 kPa is flowing through a pipe. The steam is supplied heat at constant pressure to raise its temperature to 1500C. What is the ratio of kinetic energy per unit mass of the steam to the specific enthalpy change of the steam if steam is flowing at 100 m/s? Ans. (100)^2/(2776.6-2675.6)/2000=5% In steady flow devices like turbines, compressors/pumps, heat exchangers, throttling devices it is usually a good approximation to neglect flow kinetic energy (or kinetic energy changes). Exception: nozzles and diffusers. SFEE applied to compressors m ( hout hin V Usually V 2 2 in ) Q W cv V out V in 2 hout hin 2 out 2 2 m ( hout hin ) Q W cv m ( hout hin ) 0 SFEE for liquid nozzles, pumps and water turbine: toward the Bernoulli equation m ( ho u t hin V 2 out V 2 in 2 g ( z out z in )) Q W c v Further assumptions: A. Incompressible substance, B. Negligible heat transfer C. negligible friction despite “the rubbing liquid layers” D. B and C leading to near isothermal operation. hout hi n v ( p o ut p in ) W cv 2 2 V out V in m v ( p out p in ) g ( z out z in ) 2 2 2 p o u t V out p in V in m ( g z out ) ( g z in ) 2 2 SFEE applied to heat exchangers hot (h) cold (c) m c hc , out m h hh , out m c hc ,in m h hh ,in Take CV enclosing the stream that is hot at inlet m h ( h h , o u t h h , in ) Q Take CV enclosing the stream that is cold at inlet m c ( h c , o u t hc , in ) Q Mixing chambers or “direct contact heat exchangers” 3 2 1 m 3 m1 m 2 Conservation of mass m 1 h1 m 2 h 2 m 1 m 2 h3 Conservation of energy