SignificantFigur
advertisement

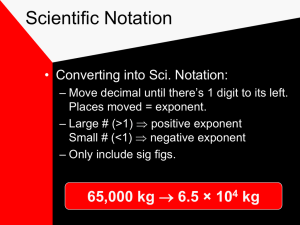
Starter 1. The radius of the moon is 1,737,000 meters. Write this in scientific notation. 2. The diameter of a carbon atom is 0.000000000154 meters. Write this in scientific notation. 3. Convert the following numbers into standard notation: a. 6.7 x 106 b. 8.8 x 10-4 Starter 1. The radius of the moon is 1,737,000 meters. Write this in scientific notation. 6 1.737 x 10 meters 2. The diameter of a carbon atom is 0.000000000154 meters. Write this in scientific notation. 1.54 x 10-10 meters 3. Convert the following numbers into standard notation: a. 6.7 x 106 6,700,000 b. 8.8 x 10-4 0.00088 Measurement • A quantity that has both a number and a unit. • Fundamental to the experimental sciences. Accuracy, Precision, and Error • Accuracy- How close a measure comes to the actual value of whatever is measured. • Precision- A measure of how close a series of measurement are to one another. XXX XXX X Error X X X If you boil water and the thermometer reads 99.1 C . You know that the boiling point is 100. C. What is the error? X X Error = Experimental Value – Accepted Value Error • Accepted Value = 100 • Experimental value = 99.1 Error = 99.1 – 100 = - 0.9 C Percent Error = Error Accepted Value Significant Figures Sig Figs • Measurements must always be reported to the correct number of significant figures because calculated answers depend on the number of sig figs used in the calculations. Significant Figures How would you record the volume of liquid in this graduated cylinder? Volume = 9.6 mL Could I have recorded the volume as 9.65 mL? What about 10 mL? What are Significant Figures? Significant figures are the digits in a measurement that contribute to the precision. I promise this will make more sense after a few examples! The number of significant figures in a measurement represents how precise that measurement is. For example, a measurement of 3.1 g (2 sig figs) is less precise than a measurement of 3.12 g (3 sig figs). Comparison This graduated cylinder reads: 42.9 mL This beaker reads: 23 mL Which is more precise?? Counting Significant Figures Scientists need to be able to count the number of significant figures in a number. There are rules to do this. Rule 1 Any digit that is not zero is significant. Examples: 65.2 has 3 sig figs 7.896735 has 7 sig figs 7,324 has 4 sig figs Rule 2 Zeros that appear between any two non-zero digits are significant. Examples: 504.2 has 4 sig figs 3004.28 has 6 sig figs 6.70809 has 6 sig figs Rule 3 Leading zeros are not significant. These zeros are sometimes called placeholders. Examples: Leading zeros 0.00005 has 1 sig fig 0.0234 has 3 sig figs 0.0000007081 has 4 sig figs Remember, this zero is significant! All these numbers have 2 sig figs! 52 5.2 0.52 0.052 0.0052 0.00052 Rule 4 Trailing zeros in numbers containing decimals are significant. Examples: trailing zeros 0.5100 has 4 sig figs 0.234000 has 6 sig figs 0.0071200 has 5 sig figs Leading zeros are not significant Rule 5 Trailing zeros in numbers that do not contain a decimal are not significant. Examples: trailing zeros 5100 has 2 sig figs 232,000 has 3 sig figs 70,000 has 1 sig fig 70,000. has 5 sig figs Significant Figures in Scientific Notation For numbers in scientific notation, determine the number of sig figs by looking at the decimal number. Examples: 5.100 x 103 has 4 sig figs 2.3 x 1026 has 2 sig figs 7.0000 x 10-4 has 5 sig fig