PPT: Measurement and Conversions
advertisement

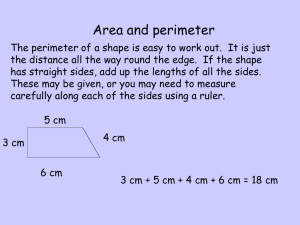
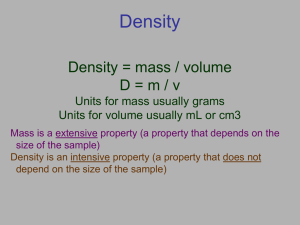
Unit 1: Measurement and Conversions http://old.unit5.org/roller Scientific Notation Review Often used to express very large or very small numbers. Also used to maintain correct number of significant figures. Form: (# from 1 to 9.999) x 10exponent 800 = 8 x 10 x 10 = 8 x 102 2531 = 2.531 x 10 x 10 x 10 = 2.531 x 103 0.0014 = 1.4 / 10 / 10 / 10 = 1.4 x 10-3 Scientific Notation Practice Change the given number to standard form. 000000187000000 1.87 x 10–5 = 0.0000187 3.7 x 108 = 370,000,000 7.88 x 101 = 78.8 2.164 x 10–2 = 0.02164 (-) exponent = number < 1 (+) exponent = number > 1 Scientific Notation Practice Change the given number into scientific notation. 12,340 = 0.369 = 0.008 = 1,000,000,000 = 1.234 x 104 3.69 x 10–1 8 x 10–3 1 x 109 Significant Figures about… A student is combining separate water samples, all of differing volumes, into one large bucket. Samples A, B and C are 25.5 mL, 16.37 mL and 51 mL, respectively. Once combined, what is the total volume of all the samples? 92.87 mL NO! Because the samples were each measured with a different level of precision, we must factor that into our calculations by identifying what are called significant figures. Measurement and Accuracy • The last digit of any measured number is assumed to be an estimate (uncertain) • The second to last digit is assumed to be known with certainty (based on a line) A (25.5 mL) B (16.37 mL) C (51 mL) 60 26 25 16.4 16.3 50 Identifying Significant Figures Counting SF’s in a number Non-zero numbers: ALWAYS count as SF Zeroes Relative to the non-zero numbers . Left: NEVER count as SF (0.000345) Middle: ALWAYS count as SF (5001) Right: sometimes… w/ decimal point: count as SF (25.10) w/o decimal point: DO NOT count as SF (8200) Exact Numbers: IGNORE SF Counts (28 students in this class) Constants (1 mol = 6.022 x 1023) Conversions (1 in = 2.54 cm) How many Sig Figs? Measurement Number of SF Measurement 1. 25 g 2 8. 0.12 kg 2 2. 0.030 kg 2 9. 1240560. cm 7 3. 1.240560 x 106 mg 7 10. 6000000 kg 1 4. 6 x 104 sec 1 11. 6.00 x 106 kg 3 5. 246.31 g 5 12. 409 cm 3 6. 20.06 cm 4 13. 29.200 dm 5 7. 1.050 m 4 14. 0.02500 g 4 Number of SF Sig Figs with Calculations Note: For any calculations, always perform the entire calculation without rounding, and then round the final answer. Addition/Subtraction • Round the answer to the LEAST number of decimal places found (least precise) 11.31 + 33.264 + 4.1 = 48.674 → rounded to 48.7 Multiplication/Division • Round the answer to the smallest number of SF found 5.282 x 3.42 = 18.06444 → rounded to 18.1 (3.42 only has 3 SF) Back to the original question… A student is combining separate water samples, all of differing volumes, into one large bucket. Samples A, B and C are 25.5 mL, 16.37 mL and 51 mL, respectively. Once combined, what is the total volume of all the samples? 25.5 mL + 16.37 mL + 51 mL = 92.87 mL 93 mL Could I write that as 93.0? NO! More practice with SF If you made measurements of three samples of water (128.7 mL, 18 mL and 23.45 mL), and then poured all of the water together in one, unmarked container, what total volume of water should you report? Support your answer. 128.7 mL + 18 mL + 23.45 mL = 170.15 mL 170. mL or 1.70 x 102 mL Practice with Sig Fig Calculations - 8.74 x 10 1. A 7 x 10 2. A 4.356 x 10 1.2 x 10 -6 6 3. A 5.7 9.863 4. A 20 3.73 5. A6.022 x 10 -5 - 5.1 x 10 3 .2 -4 = -6.118 x 10-9 report -6 x 10-9 (1 SF) -3 = 3.63 x 109 report 3.6 x 109 (2 SF) = 15.563 report 15.6 (tenths place) -6 = 16.27 report 20 (tens place) = 1.7225 x 10-5 report 1.7 x 10-5 (2 SF) Complete calculation, and then follow order of operations to determine how many SF would be carried for each step from Industry Week, 1981 November 30 The Metric System SI System • The International System of Units – abbreviated SI from the French Le Système international d'unités • Based on the metric system (with small variations) • Based on powers of ten – Uses prefixes to differentiate between powers • Used in nearly country except U.S. (Liberia and Myanmar are some others…) The International System of Units Quantity Name Symbol Volume Length Mass Time Amount of substance Thermodynamic temperature Electric current Luminous intensity liter meter kilogram second mole Kelvin amperes candela L m kg s mol K amps cd Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 16 Area and Volume: Derived Units Area = length x width = 5.0 m x 3.0 m = 15 ( m x m) = 15 m2 Volume = length x width x height = 5.0 m x 3.0 m x 4.0 m = 60. ( m x m x m) = 60. m3 Derived Units Commonly Used in Chemistry Quantity Area Volume Force Pressure Energy Power Voltage Frequency Electric charge Name square meter cubic meter newton pascal joule watt volt hertz coulomb Symbol m2 m3 N Pa J W V Hz C Prefixes in the SI System The Commonly Used Prefixes in the SI System Prefix Symbol Meaning Power of 10 for Scientific Notation _______________________________________________________________________ 1,000,000 106 1,000 103 mega- M kilo- k deci- d 0.1 10-1 centi- c 0.01 10-2 milli- m 0.001 10-3 micro- m 0.000001 10-6 nano- n 0.000000001 10-9 Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 118 1024 g 1021 g Quantities of Mass 1018 g 1015 g 1012 g Giga- 109 g Mega- 106 g Kilo- 103 g base 100 g milli- 10-3 g micro- 10-6 g nano- 10-9 g pico- 10-12 g femto- 10-15 g atomo- 10-18 g Ocean liner Indian elephant Average human 1.0 liter of water Grain of table salt 10-21 g 10-24 g Kelter, Carr, Scott, Chemistry A Wolrd of Choices 1999, page 25 Earth’s atmosphere to 2500 km Typical protein Uranium atom Water molecule Reporting Measurements • Must use significant figures • Report what is known with certainty Using dashes • Add ONE digit of uncertainty beyond that Using estimation The implication is that for any measurement, the last digit is an estimate and uncertain, and the next to last is known with certainty Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 46 Practice Measuring Timberlake, Chemistry 7th Edition, page 7 0 cm 1 2 3 4 5 4.5 cm 0 cm 1 2 3 4 5 4.54 cm 0 cm 1 2 3 4 5 3.0 cm Measurement/Sig Fig Practice Draw a picture showing the markings (graduations) on glassware that would allow you to make each of the following volume measurements of water and explain your answers (the numbers given are as precise as possible): a. 128.7 mL b. 18 mL c. 23.45 mL Mark every 1 mL Mark every 10 mL Mark every 0.1 mL Implied Range of Uncertainty 30 40 50 60 Implied range of uncertainty in a measurement reported as 50. cm (±5) 3 4 5 6 Implied range of uncertainty in a measurement reported as 5.0 cm (±0.5) 3 4 5 6 Implied range of uncertainty in a measurement reported as 5.00 cm (±0.05) Dorin, Demmin, Gabel, Chemistry The Study of Matter 3rd Edition, page 32 Reading a Meniscus 10 mL 10 8 proper line of sight reading correct 6 graduated cylinder 20 ? 15 ?1 mL 1.50 15.0 xmL 10 mL 10 Conversion Factors How many cm are in 1.32 meters? equality: 1 m = 100 cm (or 0.01 m = 1 cm) applicable conversion factors: ______ 1m 100 cm or 1.32 m 100 cm 1m 100 cm ______ 1m = 132 cm We use the idea of unit cancellation to decide upon which one of the two conversion factors we choose. Both ways are equally good! 1. How many kilometers is 15,000 decimeters? 15,000 dm OR… 1m 10 dm 1 km 1,000 m ( )( 1 km 1m ______ 15,000 dm ____ 10 dm 1,000 m = 1.5 km ) 2. How many seconds is 4.38 days? ( )( 24 h 4.38 d ____ 1d )( ) 60 min _____ 1h 60 s ____ 1 min = 378,432 s If we are accounting for significant figures, we would change this to… 3.78 x 105 s 3. Convert 41.2 cm2 to m2 ( ) 1m 41.2 cm2 ______ = 0.412 m2 WRONG! 100 cm = 0.412 cm.m Recall that… 41.2 cm2 = 41.2 cm.cm ( )( 41.2 cm.cm ______ 1m 100 cm ) 1m ______ 100 cm = 0.00412 m2 ( ) 1 m2 41.2 cm2 ________ = (100)2 cm2 0.00412 m2 4. Convert 41.2 cm2 to mm2 Recall that… 41.2 (1 cm)2 = (10 mm )2 ( cm2 ) 102 mm2 = _____ 1 cm2 4,120 mm2 5. Convert to 480 cm3 to m3 cm.cm.cm ( )( 1m 480 cm32 _____ 100 cm 1m _____ )( 100 cm ) 1m _____ = 100 cm or 3 ( ) ( 3 1m 480 cm3 _____ 100 cm 0.00048 m3 = or 480 cm3 ) 1m _________ 4.8 x 10-4 m3 = 1000000 cm3 Comparison of English and SI Units 1 inch 2.54 cm 1 inch = 2.54 cm Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 119 SI-US Conversion Factors Equality Conversion Factors Length 2.54 cm = 1 in. 2.54 cm 1 in and 1 m = 39.4 in. 39.4 in 1m and 946 mL = 1 qt 946 mL 1 qt and 1 qt 946 mL 1 L = 1.06 qt 1.06 qt 1L and 1L 1.06 qt and 1 lb 453.6 g and 1 kg 2.20 lb 1 in 2.54 cm 1m 39.4 in. Volume Mass 453.6 g = 1 lb 1 kg = 2.20 lb 453.6 g 1 lb 2.20 lb 1 kg Practical Conversions Teachers get a lot of grief from normal workers because they only work 36 weeks a year. How many extra hours, per day, would a teacher have to put in to match the typical worker, assuming a teacher works 8 hrs per day for those 36 weeks? What assumptions must we make? Density Review how tightly packed the particles are Density = mass volume D m m V D Typical units: g/cm3 for solids V g/mL for fluids liquids and gases Glass: liquid or solid? Monty Python’s take on analytical science and density with regard to witches… Density Review 1. A sample of lead (Pb) has mass 22.7 g and volume 2.0 cm3. Find sample’s density. m D D V m V 22.7 g 2.0 cm 3 = 11 g cm 2. Another sample of lead occupies 16.2 cm3 of space. Find sample’s mass. V m = D V 11 g cm 3 16.2 cm 3 = 180 g 3 More Density Review Problems… 3. A 119.5 g solid cylinder has radius 1.8 cm and height 1.5 cm. Find sample’s density. 4. A 153 g rectangular solid has edge lengths 8.2 cm, 5.1 cm, and 4.7 cm. Will this object sink in water? m 3. A 119.5 g solid cylinder has radius 1.8 cm and height 1.5 cm. Find sample’s density. 1.8 cm 1.5 cm m D V D m V = p r2 h 2 SF = p (1.8 cm)2(1.5 cm) = 15.2681 cm3 V 119.5 g 15.2681 cm 3 = 7.8 g cm 3 8.2 cm 4. A 153 g rectangular solid has edge lengths 8.2 cm, 5.1 cm, and 4.7 cm. Will this object sink in water? 5.1 cm 4.7 cm (Find object’s density and compare it to water’s density.) 2 SF V=lwh m D D m V = 8.2 cm (5.1 cm)(4.7 cm) V = 196.554 cm3 153 g 196.554 cm 3 = 0.78 g cm 3 <1 No; it floats. Will bowling balls sink or float in H2O? If DBB > 1, it will sink If DBB < 1, it will float 21.6 cm in diameter Vsphere = 4/3 p r3 V = 4/3 p (10.8 cm)3 V = 5,276.7 cm3 m D m=DV V m = (1.0 g/cm3)(5276.7 cm3) m = 5276.7 g Since the mass of a BB varies, let’s figure out at what mass it will sink v. float …or 11.6 lbs Measurements Metric (SI) units Length Mass Volume Density Timberlake, Chemistry 7th Edition, page 40 Prefixes Uncertainty Conversion factors Significant figures Problem solving with conversion factors