Lewis Structures
advertisement

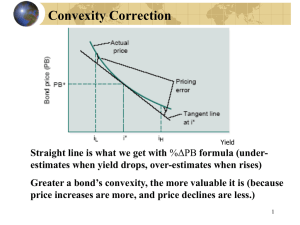
Bonding Review Covalent Bonds (2 nonmetals) …atoms share e– to get a full valence shell C 1s2 2s2 2p2 4 valence e1s2 2s2 2p5 F 7 valence e- *Both need 8 v.e – for a full outer shell (octet rule)!* xx o o C o o x x Fx xx Draw the Lewis dot structure for the following elements (write e- config first): Si 1s2 2s2 2p6 3s2 3p2 4 valence e- O 1s2 2s2 2p4 6 valence e- P 1s2 2s2 2p6 3s2 3p3 5 valence e- B 1s2 2s2 2p1 3 valence e- Ar 1s2 2s2 2p6 3s2 3p6 8 valence e- Br 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 7 valence e- Notice any trends…? 1 2 3 4 5 6 7 H 8 He Be Na Mg K Ca Rb Sr Cs Ba TRANSITION METALS Li B C N O F Ne Al Si P S Cl Ar Se Br Kr Te I Xe The group # corresponds to the # of valence e– Let’s bond two F atoms together… Each F has 7 v. e– and each needs 1 more e– F F F F F2 Now let’s bond C and F atoms together… carbon tetrafluoride (CF4) F F C F F F C F F F Lewis Structures: 2D Structures NH3 CH2O H N H H CO2 H H C H H CH4 O C O (0) (0) (0) O S SO2 O Drawing Lewis Structures 1. Sum the # of valence electrons from all atoms Anions: add e– (CO32- : add 2 e– ) 2. Cation: subtract e– (NH4+: minus 1 e– ) Predict the arrangement of the atoms • Usually the first element is in the center (often C, never H) 3. 4. Make a single bond (2 e–) between each pair of atoms Arrange remaining e– to satisfy octets (8 e– around each) • Place electrons in pairs (lone pairs) • Too few? Form multiple bonds between atoms: double bond (4 e– ) and triple bond (6 e– ) 5. Check your structure! All electrons have been used All atoms have 8e- Exceptions: Remember that H only needs 2e– ! Lewis Structure Practice Draw a Lewis Structure for the following compounds: • CH4 • H2O • NF3 • OF2 O H H F Br H C N O N F F F • HCN H F • HBr O • NO3- N O • CO3 O O 2- C O O Lewis Structure Trends Here are some useful trends… C group O • Forms a combo of 4 bonds and no LP (Lone Pairs) • i.e. CO2 N group • Forms a combo of 3 bonds and 1 LP • i.e. NH3 O group H N H • Forms a combo of 2 bonds and 2 LP • i.e. CH2O F group (halogens) • Forms 1 bond and 3 LP • i.e. OF2 O F ) 0 ( O (0 H F Note that these are NOT always true! ) 0 ( C ) Carbonite CO22Carbonate? CO32- Resonance Structures Resonance structures differ only in the position of the electrons Show resonance O C O O Show movement of e- C O O O C O O O • The actual structure is a hybrid (average) of the resonance structures • Technically NOT two single bonds and one double bond • All 3 Oxygen atoms share the double bond • 3 equal bonds (somewhere between a double and single) Arrow formalism: curved arrows show electron movement Predicting Molecular Shape: VSEPR (Valence Shell Electron Pair Repulsion) • Electrons repel each other • The molecule adopts a 3-D shape to keep the electrons (lone pairs and bonded e-) as far apart as possible • Different arrangements of bonds/lone pairs result in different shapes • Shapes depend on # of bonds/lone pairs (“things”) and LP around the central atom Selected Shapes and Geometries using VSEPR “Things” Carbon Dioxide: CO2 Lewis Structure O C O (0 ) (0 ) (0 ) O C O Two “things” (bonds or lone pairs) Linear geometry 0 LP → Linear Shape 180o Bond angle Formaldehyde: CH2O Lewis Structure O O C H C H H Three “things” Trigonal planar geometry 0 LP → Trigonal planar shape 120° bond angles H Sulfur Dioxide: SO2 Lewis Structure O S A S O B O A Three “things” Trigonal planar geometry 1 LP → Bent shape 120° bond angles O A Methane: CH4 Lewis Structure H H C H H Four “things” (bonds/LP) Tetrahedral geometry 0 LP → Tetrahedral shape 109.5o bond angles Ammonia: NH3 Lewis Structure H N H H Four “things” (bonds/LP) Tetrahedral geometry 1 LP → Trigonal pyramid shape 107o bond angles Water: H2O Lewis Structure 4 “things” (bonds/LP) O H H Tetrahedral Geometry 2 LP → Bent Shape 104.5o bond angle Hydrogen Chloride: HCl Lewis Structure H Cl Four “things” (bonds/LP) Tetrahedral geometry 3 LP → Linear Shape H Cl Cl No Bond angle A special note… For any molecule having only two atoms… e.g. N2, CO, O2, Cl2, HBr, etc. N N O O Cl Cl H Br Geometry = Linear Shape = Linear Bond Angle(s)? = None It is much like geometry… what is formed by connecting two points? …a line. You will need to commit these to memory! “Things” VSEPR Practice (w/o aid of yellow sheet) • CO2 • CH3COO- G: S: Angle: G: S: Angle: • ClO2- • PBr3 G: S: Angle: G: S: Angle: • NO2- • AsO43- G: S: Angle: G: S: Angle: Electronegativity and Bond Type The electronegativity difference between two elements helps predict what kind of bond they will form. Electronegativity Bond type difference ≤ 0.4 0.5 – 1.8 > 1.8 Definition Covalent e- are evenly shared Polar covalent e- are unevenly shared Ionic e- are exchanged (gained or lost) Practice with Bond Types Sample Bonds Electronegativity Difference Bond Type? Ionic NaCl 3.0 – 0.9 = 2.1 Covalent Cl-Cl 3.0 – 3.0 = 0 Polar covalent C-O 3.5 – 2.5 = 1.0 Covalent C-H 2.5 – 2.1 = 0.4 H 2.1 Li 1.0 Na 0.9 K 0.8 Be 1.5 Mg 1.2 Ca 1.0 B 2.0 Al 1.5 C 2.5 Si 1.8 N 3.0 P 2.1 O 3.5 S 2.5 F 4.0 Cl 3.0 Br 2.8 I 2.5 Electronegativity difference ≤ 0.4 0.5 – 1.8 > 1.8 Bond type Covalent Polar covalent Ionic Dipole Moments and Polarity • Occurs in polar covalent bonds • Uneven distribution of e• Atoms become partially charged Partially “+” charged end δ+ H Cl δ- Arrow points toward partially “-” end Polarity Examples 1. Check molecule for dipole moments (polar bonds) 2. When determining overall polarity, an imbalanced structure will likely be polar (at least partially) 3. Even with polar bonds, a balanced structure is non-polar overall 4. Any structure with lone pairs on the central atom is automatically polar! Try these with your neighbors… • • • • • HCN CO2 CO32CH2O SO2 Polar Non-polar Non-polar Polar Polar • • • • • CH4 CH3F C3H8 CO NH3 Non-polar Polar Non-polar Polar Polar