Energy and Heat Test Review - High School Chemistry
advertisement

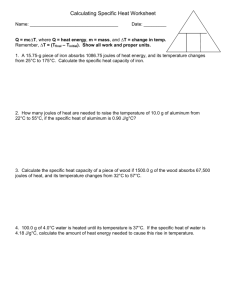
Energy and Heat Test Review 1. The study of heat changes that occur during chemical reactions is called _thermochemistry_____________________ 2. Explain the difference between heat and temperature: Temperature is a measure of how much an energy a substance may have; Heat is the transfer of energy due to a difference in temperature 3. ____Energy___________ is the the capacity to do work or supply heat 4. Contrast exothermic and endothermic – Exothermic – heat is transferred from the system to its surroundings; Endothermic – heat is transferred from the surroundings to the system 5. Two units of heat measurement are __Joules____ and _calories_________. The conversion factor is __4.184 J = 1 calorie________________. 6. How much heat it takes to raise the temperature of an object by 1oC is called _____heat capacity_________________. The two factors that influence this are ___type_________ and ___amount of substance______. 7. The amount of energy required to raise the temperature of 1g of substance by 1oC is __specific heat___ and the formula is __q=mCp∆T_____________ 8. ___Calorimetry______________ is using the specific heat of something you know to find the specific heat of something you don’t 9. Explain the Law of Conservation of Energy: Heat lost by one substance must be equal to that gained by another substance 10. From the below heat curve, match the letter to the following terms: Solid A Freezing K Melting D Liquid E Condensing J Vaporization H Melting Point B Gas I Heat of Fusion C Boiling Point F Heat of Vaporization G 11. Fill in the correct terms on the diagram below 12. What is the specific heat of a metal if a 40g sample at 110.0oC raises the temperature of 300g of water from 23.2oC to 29.1oC? q=300(4.184)(5.9)= 7405.68 Cp=-7405.68/(40*(29.1-110))= 2.26 13. What is the specific heat of a substance if a 45g sample at 101.5oC raises the temperature of 154g of water from 20.6oC to 33.1oC? q=154(4.184)(12.5)=8054.2 Cp=-8054.2/(45*(33.1-101.5))= 2.62 14. Granite has a specific heat of 800 J/g∙°C. What mass of granite is needed to store 150,000 J of heat if the temperature of the granite is to be increased by 15.5°C? m=150000/(800*15.5)= 12.1g 15. A total of 54.0 joules of heat are absorbed as 58.3 g of lead is heated from 12.0°C to 42.0°C. From these data, what is the specific heat of lead? Cp=54/(58.3*30)= .03 16. How much energy is required to heat 120.0 g of water from 2.0 °C to 24.0 °C? (Cp of H2O = 4.184 J/g °C) q=120(4.184)(22)=11045.76 17. To what temperature will a 50.0 g piece of glass raise if it absorbs 5275 joules of heat and its specific heat capacity is 0.50 J/g°C? The initial temperature of the glass is 20.0°C ∆T=5275/(50*.5)= 211 Final temperature = 20+211=231 18. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°C to 55°C, if the specific heat of aluminum is 0.90 J/g°C? q=10(.9)(33)=297 19. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°C to 55°C, if the specific heat of aluminum is 0.90 J/g°C? q=10(33).9=297 20. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes from 32°C to 57°C. Cp=67500/(1500*25)=.19