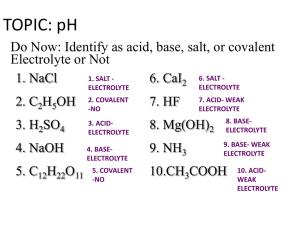
[H3O+], [OH–], pH - BC Learning Network
advertisement
![[H3O+], [OH–], pH - BC Learning Network](http://s2.studylib.net/store/data/005610503_1-47ff8b2322e271c154ebb4db3f00887e-768x994.png)
Working with + – [H3O ], [OH ], pH, and pOH Introduction Here, we’ll introduce some useful relationships that exist among hydronium concentration, hydroxide concentration, pH, and pOH From Math: Here’s something that we should be aware of from Math, concerning logs From Math: If: If A TIMES B is equal to c a × b = c From Math: If: a × b = c Then: log(a) + log(b) = log(c) Then the log of (a) PLUS the log of (b) is equal to the log of (c). H 3O OH log H 3O log OH Kw log K w log H 3O log OH log K w pH pOH pK w We already know that in any aqueous solution the concentration of hydronium times the concentration of hydroxide is equal to Kw. H 3O OH log H 3O log OH Kw log K w log H 3O log OH log K w pH pOH pK w If we take the log of everything, it follows that the log of H3O+ concentration plus the log of OH minus concentration is equal to the log of Kw. H 3O OH –1 log H 3O log OH Kw log K w log H 3O log OH log K w pH pOH pK w Now, we’ll multiply everything by negative 1 H 3O OH log H 3O log OH Kw log K w log H 3O log OH log K w pH pOH pK w And we get that the negative log of hydronium ion concentration plus the negative log of hydroxide ion concentration is equal to the negative log of Kw. H 3O OH log H 3O log OH Kw log K w log H 3O log OH log K w pH pOH pK w The negative log of the hydronium ion concentration is the pH, H 3O OH log H 3O log OH Kw log K w log H 3O log OH log K w pH pOH pK w the negative log of the hydroxide ion concentration is pOH, H 3O OH log H 3O log OH Kw log K w log H 3O log OH log K w pH pOH pK w And the negative log of Kw is equal to something called pKw. H 3O OH Kw pH pOH pK w So we have two important equations: the concentration of hydronium times the concentration of hydroxide is equal to Kw. H 3O OH Kw pH pOH pK w And the pH PLUS the pOH is equal to pKw. H 3O OH Kw pH pOH pK w Where pKw = –logKw Where the pKw is defined as the –logKw H 3O OH K w pH pOH pK w True for any temperature at which water is a liquid Both of these equations are true for ANY temperature at which water is a liquid. 25°C Now, we’ll zoom into a temperature of 25°C. At 25°C Kw = 1.0 × 10-14 pKw = –logKw = –log(1.0 × 10–14) = 14.00 At 25°C, Kw = 1.0 × 10-14 At 25°C Kw = 1.0 × 10-14 pKw = –logKw = –log(1.0 × 10–14) = 14.00 So at 25°C, the pKw… At 25°C Kw = 1.0 × 10-14 pKw = –logKw = –log(1.0 × 10–14) = 14.00 Which is the negative log of Kw At 25°C Kw = 1.0 × 10-14 pKw = –logKw = –log(1.0 × 10–14) = 14.00 Is the –log of 1.0 × 10–14 … At 25°C Kw = 1.0 × 10-14 pKw = –logKw = –log(1.0 × 10–14) = 14.00 Which is equal to 14.00 At 25°C pKw = 14.00 So we can say that specifically at 25°C At 25°C pKw = 14.00 The pKw is equal to 14.00 At 25°C pKw = 14.00 pKw = pH + pOH = 14.00 Remember, we had recently determined that pKw is equal to pH + pOH At 25°C pKw = 14.00 pKw = pH + pOH = 14.00 And at 25° pkw = 14.00 At 25°C pH + pOH = 14.00 Therefore we can say that at 25°C, pH + pOH = 14.00 At 25°C This is true ONLY at 25°C! pH + pOH = 14.00 You’ll be using this equation a lot. Just make sure you use caution. Remember, this is true ONLY at 25°C. At 25°C If temperature is not mentioned, assume that it is 25°C Remember that if temperature is not mentioned in a problem, we can assume that it is 25°C At 25°C If temperature is not mentioned, assume that pH + pOH = 14.00 And we can assume that pH + pOH is equal to 14. The pOH of a solution is 3.49. What is the pH? pH + pOH = 14.00 Here’s an example. We’re told that the pOH of a solution is 3.49 and we’re asked what the pH is? The pOH of a solution is 3.49. What is the pH? pH pOH 14.00 pH 14.00 pOH 14.00 3.49 pH 10.51 pH + pOH = 14.00 We are not given the temperature, so we can assume its 25°C and that pH + pOH is equal to 14 The pOH of a solution is 3.49. What is the pH? pH pOH 14.00 pH 14.00 pOH 14.00 3.49 pH 10.51 pH + pOH = 14.00 We want to find the pH, so we rearrange the blue equation to solve for pH, and we get the yellow equation: pH = 14 minus pOH. The pOH of a solution is 3.49. What is the pH? pH pOH 14.00 pH 14.00 pOH 14.00 3.49 pH 10.51 pH + pOH = 14.00 Which is 14 minus 3.49 The pOH of a solution is 3.49. What is the pH? pH pOH 14.00 pH 14.00 pOH 14.00 3.49 pH 10.51 pH + pOH = 14.00 And that equals 10.51. So the pH is 10.51. At ANY Temperature ONLY at 25°C Now we’ll review the things we know are true at any temperature and things we know are true ONLY at 25°C. At ANY Temperature ONLY at 25°C We’ll start with equations that are true at ANY temperature At ANY Temperature [H3O+][OH–] = Kw [H+][OH–] = Kw ONLY at 25°C At ANY Temperature [H3O+][OH–] = Kw pH + pOH = pKw pH + pOH = pKw ONLY at 25°C At ANY Temperature [H3O+][OH–] = Kw pH + pOH = pKw pH = –log[H3O+] pH = –log[H3O+] ONLY at 25°C At ANY Temperature [H3O+][OH–] = Kw pH + pOH = pKw pH = –log[H3O+] [H3O+] = 10–pH [H3O+] = 10–pH ONLY at 25°C At ANY Temperature [H3O+][OH–] = Kw pH + pOH = pKw pH = –log[H3O+] [H3O+] = 10–pH pOH = –log[OH–] pOH = –log[OH–] ONLY at 25°C At ANY Temperature [H3O+][OH–] = Kw pH + pOH = pKw pH = –log[H3O+] [H3O+] = 10–pH pOH = –log[OH–] [OH–] = 10–pOH [OH–] = 10–pOH ONLY at 25°C At ANY Temperature [H3O+][OH–] = Kw pH + pOH = pKw pH = –log[H3O+] [H3O+] = 10–pH pOH = –log[OH–] [OH–] = 10–pOH pKw = –log(Kw) pKw = –log(Kw) ONLY at 25°C At ANY Temperature ONLY at 25°C [H3O+][OH–] = Kw pH + pOH = pKw pH = –log[H3O+] [H3O+] = 10–pH pOH = –log[OH–] [OH–] = 10–pOH pKw = –log(Kw) Kw = 10–pKw We can solve the previous equation for Kw, we get Kw = 10–pKw At ANY Temperature [H3O+][OH–] = Kw pH + pOH = pKw pH = –log[H3O+] [H3O+] = 10–pH pOH = –log[OH–] [OH–] = 10–pOH pKw = –log(Kw) Kw = 10–pKw Now we’ll review what is true ONLY at 25°C ONLY at 25°C At ANY Temperature ONLY at 25°C [H3O+][OH–] = Kw [H3O+][OH–] =1.0 × 10–14 pH + pOH = pKw pH = –log[H3O+] [H3O+] = 10–pH pOH = –log[OH–] [OH–] = 10–pOH pKw = –log(Kw) Kw = 10–pKw [H+][OH–] = 1.0 × 10–14 At ANY Temperature ONLY at 25°C [H3O+][OH–] = Kw [H3O+][OH–] =1.0 × 10–14 pH + pOH = pKw pH + pOH = 14.00 pH = –log[H3O+] [H3O+] = 10–pH pOH = –log[OH–] [OH–] = 10–pOH pKw = –log(Kw) Kw = 10–pKw pH + pOH = 14.00 At ANY Temperature ONLY at 25°C [H3O+][OH–] = Kw [H3O+][OH–] =1.0 × 10–14 pH + pOH = pKw pH + pOH = 14.00 pH = –log[H3O+] Kw = 1.0 × 10–14 [H3O+] = 10–pH pOH = –log[OH–] [OH–] = 10–pOH pKw = –log(Kw) Kw = 10–pKw Kw = 1.0 × 10–14 At ANY Temperature ONLY at 25°C [H3O+][OH–] = Kw [H3O+][OH–] =1.0 × 10–14 pH + pOH = pKw pH + pOH = 14.00 pH = –log[H3O+] Kw = 1.0 × 10–14 [H3O+] = 10–pH pKw = 14.00 pOH = –log[OH–] [OH–] = 10–pOH pKw = –log(Kw) Kw = 10–pKw pKw = 14.00. At ANY Temperature ONLY at 25°C [H3O+][OH–] = Kw [H3O+][OH–] =1.0 × 10–14 pH + pOH = pKw pH + pOH = 14.00 pH = –log[H3O+] Kw = 1.0 × 10–14 [H3O+] = 10–pH pKw = 14.00 pOH = –log[OH–] [OH–] = 10–pOH pKw = –log(Kw) Kw = 10–pKw So we see that any equations that contain the number 14, are ONLY true at 25°C. At ANY Temperature ONLY at 25°C [H3O+][OH–] = Kw [H3O+][OH–] =1.0 × 10–14 pH + pOH = pKw pH + pOH = 14.00 pH = –log[H3O+] Kw = 1.0 × 10–14 [H3O+] = 10–pH pKw = 14.00 pOH = –log[OH–] You REALLY need to KNOW all these equations! [OH–] = 10–pOH pKw = –log(Kw) Kw = 10–pKw In order to succeed in the rest of this unit You REALLY need to KNOW all these equations! Pause and make a screen capture of this, save it, and go over it periodically. [H3 O +] [H3O+][OH–] = 1.00 × 10–14 pH = –log[H3O+] pOH = –log[OH–] [H3O+] = 10–pH pH [OH–] [OH–] = 10–pOH pH + pOH = 14.00 pOH Here’s the “square” at 25°C. It shows all the formulas you can use to make one step or two step conversions among [H3O+], [OH-], pH, and pOH. [H3 O +] [H3O+][OH–] = 1.00 × 10–14 pH = –log[H3O+] pOH = –log[OH–] [H3O+] = 10–pH pH [OH–] [OH–] = 10–pOH pH + pOH = 14.00 pOH It would be good if you could draw something similar to this from memory. It will help you with the calculations you’ll be required to do. [H3 O +] [H3O+][OH–] = 1.00 × 10–14 pH = –log[H3O+] pOH = –log[OH–] [H3O+] = 10–pH pH [OH–] [OH–] = 10–pOH pH + pOH = 14.00 pOH For now, you may want to pause the video, take a screen shot and print yourself a copy of this to work with. [H3O+] [OH–] pH pOH Here’s a simpler version we can use to help us come up with plans for calculations [H3O+] [OH–] Given ? pH pOH For example, Let’s say we’re given the hydronium ion concentration and we want to find the pOH [H3O+] [OH–] Given ? pH pOH We can do this in two steps. We could start (click) by converting hydronium ion concentration of pH… [H3O+] [OH–] Given ? pH And in the second step (click), we’ll convert pH to pOH. pOH [H3O+] [OH–] Given ? pH pOH Alternately, we could have started by converting (click) hydronium concentration to hydroxide concentration [H3O+] [OH–] Given ? pH pOH And then (click) hydroxide ion concentration to pOH. This would give us the same answer as the other method. [H3O+] [OH–] ? Given pH pOH In another example, let’s say we’re given the pH and we want to find hydroxide ion concentation. [H3O+] [OH–] Given pH We could start (click) by converting pH to pOH pOH ? [H3O+] [OH–] Given pH And then (click) pOH to hydroxide concentration pOH ? [H3O+] [OH–] ? Given pH pOH Or alternately, we could have started with pH (click) and converted to hydronium concentration [H3O+] [OH–] Given pH pOH And then from (click) hydronium concentration to hydroxide concentration. ?