MCP-1

Serial Measurement of Monocyte
Chemoattractant Protein-1 After Acute
Coronary Syndromes
Results From the A to Z Trial
J Am Coll Cardiol 2007;50:2117-24
JA de Lemos, DA Morrow, SA Wiviott,
P Jarolim, MA Blazing, MS Sabatine,
RM Califf, E Braunwald
Monocyte Chemoattractant Protein-1 (MCP-1)
MCP-1 figure
• Transgenic MCP-1 mice: athero
• Plasma MCP-1 assoc with ASHD risk factors
• older age, DM, HTN, Fam Hx CAD,
LDL,
renal fxn
• Modified by preventive rx (statin, TZD, etc)
15%
MCP-1 and Outcomes After ACS
MCP1 > 238 pg/mL
10% p=0.001
MCP1 < 238 pg/mL
5%
0%
0 100 200
Days from Presentation with ACS de Lemos et al. Circulation 2003;107:690-5
300
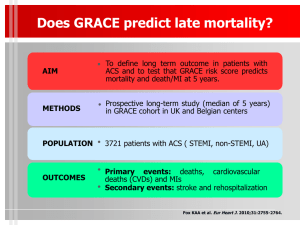
Objectives
• Evaluate the Prognostic Value of MCP-1 in
Patients following ACS
• Serial measurement in acute and chronic phase
• Account for standard risk variables
• Account for emerging biomarkers (CRP, BNP)
• Determine the influence of statin therapy on
MCP-1 levels
• Determine whether MCP-1 helps to identify candidates for more intensive statin rx
Study Design
S40
Simvastatin 80 mg
Early
Intensive
Delayed more
Conservative
Simvastatin 40 mg
N = 4497
Placebo
Placebo Placebo
Simvastatin 20 mg
Randomization Mo 1 Mo 4
Mo 24
Methods
• MCP-1 measured from baseline (n=4244), 4 mo
(n=3603) and 12 mo samples (n=2950)
• BNP (Bayer) and CRP (Denka Seiken) measured on baseline and 4 mo samples
• Endpoints compared using MCP-1 quartiles and prespecified threshold of 238 pg/mL
• Landmark analysis used to evaluate association between 4 month lab values and subsequent outcomes
Influence of Treatment Assignment
300
250
200
150
100
P=0.005
Placebo/Simvastatin 20 mg
Simvastatin 40/80 mg
50
0
Baseline 4 months 12 months
Baseline Levels of MCP-1 and Mortality
10
8
6
4
2 p<0.0001
0 120 240 360 480
Days after Index ACS Event
600 720
Quartile 3
Quartile 4
Quartile 2
Quartile 1
20
16
12
8
4
0
0
Association Between MCP-1 and
Primary Z Phase Endpoint
P<0.0001 for trend p<0.0001
120 240 360 480
Days After Index ACS Event
600 720
Quartile 4
Quartile 3
Quartile 2
Quartile 1
Baseline MCP-1 and Mortality
8
6
4
2
MCP-1 > 238 pg/mL
MCP-1 < 238 pg/mL p<0.0001
0 120 240 360 480
Days Following Randomization
600 720
MCP-1, CRP, and Mortality
p<0.0001
10
8
2
0
6
4
N=1266 p<0.001
n=924 n=1029 n=676
CRP high CRP low
> 15 mg/L < 15 mg/L
MCP-1 high
MCP-1 low
MCP-1, BNP, and Mortality
20 p=0.08
15 n=353
10
5
0 n=253 n=1605
P<0.0001
n=2030
MCP-1 high
MCP-1 low
BNP high BNP low
> 80 pg/mL < 80 pg/mL
Multivariable Analyses (Baseline)
MCP-1 > 238 pg/mL
Death
MI
Death/MI
Death/MI/CHF
Z phase primary
0.25
0.5
1 2 4
Adjusted for age, sex, weight, prior MI, ACEI, DM, smoking, index dx, rx assignment, ClCr, LDL, CRP, BNP
5
4
3
2
1
4 month MCP-1 and Mortality
MCP-1 > 238 pg/mL
MCP-1 < 238 pg/mL p<0.01
120 240 360 480
Days Following Randomization
600 720
Multivariable Analyses (4 mos)
MCP-1 > 238 pg/mL
Death
MI
Death/MI
Death/MI/CHF
Z phase primary
0.25
0.5
1 2 4
Adjusted for age sex, DM, smoking, index dx, rx assignment,
4 mo LDL, CRP, BNP
20
15
Multiple-Marker Strategy at Baseline
MCP-1, CRP, BNP
17.3
p<0.0001
10 8.3
5
4.1
1.3
0
0 1 2 3
Number of Elevated Biomarkers n= 631 2017 1290 254
Adjusted HR 1 (ref) 2.3 4.4 7.6
15
Multiple-Marker Strategy at 4 mos
MCP-1, CRP, BNP
13.3
p<0.0001
10
5.7
5
3.1
1.2
0
0 1 2 3
Number of Elevated Biomarkers n= 851 1823 845 71
Adjusted HR 1 (ref) 2.2 4.1 7.2
HR Comparing Intensive vs Conservative
Simvastatin Groups
Baseline
Death
Death/MI/CHF
Z phase Primary
> 238 pg/mL
< 238 pg/mL
> 238 pg/mL
< 238 pg/mL
> 238 pg/mL
< 238 pg/mL
Death
Death/MI/CHF
Z phase Primary
> 238 pg/mL
< 238 pg/mL
> 238 pg/mL
< 238 pg/mL
> 238 pg/mL
< 238 pg/mL
0.25 0.5 1.0 2
4 mo
Conclusions
• In pts stabilized following ACS,
MCP-1
• Associated with risk for death and major CV events
• Independent of standard clinical variables, LDL,
CRP, and BNP
• Similar findings in acute and chronic phase
• Statins had only a modest effect on MCP-1 levels
• MCP-1 did not predict benefit from early intensive statin rx
• MCP-1 merits further study
• as a risk marker in ACS
• as a target for therapy