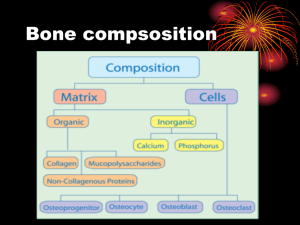
Mechanical Stimulation of Fracture Callus Cells Fracture healing
advertisement
Biomechanics Team Member:Chin Lin Wang Chung Chun Lin Ken Chung Chen 2010/12/30 Basic Orthopaedic Biomechanics and Mechano-Biology, 3rd Edition Editors: Mow, Van C.; Huiskes, Rik Copyright ©2005 Lippincott Williams & Wilkins Biomechanics of Fracture Fixation and Fracture Healing Contents 1 Mechanical Principles of Fracture Fixation 2 Biomechanics of Fracture Healing 3 Biomechanical Monitoring of Fracture Healing Introduction Up to the nineteenth century, fracture treatment was performed by external splinting Alignment Stabilize External loading and muscle activity Tissue strain and the cellular reaction As a consequence, the operative treatment of fractures, which involved new fixation systems and implants, was developed in the twentieth century Mechanical Principles of Fracture Fixation Splinting Plaster Cast and Brace External Fixation Internal Fixation Intramedullary Nail Compression Plate Lag Screws Tension band systems Plaster Cast and Brace The classical way to stabilize a fracture is plaster cast fixation. Instability due to bending movements and torque must be limited by a good fit between the plaster cast and the outer shape of the extremity. External Fixator When a fracture is accompanied by an open soft-tissue wound, a plaster cast or brace is often not possible. In such cases, an external stabilization of the fracture can be performed by using an external fixator. The distance between two connecting bars (L3) and the distance between two screws in one fragment (LS). The greater the distance, the higher the stability. Bilateral open tibia fractures stabilized by two external fixators. Left leg: monolateral double tube fixator; right leg: biplanar external fixator. There are limitations. From a biological point of view, it cannot be proposed to increase the number or the diameter of the screws as much as possible to achieve maximal stability. External fixator with four 5mm screws showed interfragmentary movements of 1 to 3 mm under partial axial loading (300 N). Intramedullary Nail Intramedullary nailing is a generally accepted internal splinting technique. The conventional Küntscher nail is a longitudinally slotted tube that is inserted into a long bone under prestress. The reaming of the bone can cause a considerable rise in intramedullary pressure and temporary damage to the bone's blood supply . Interfragmentary Compression Interfragmentary compression (N) created by a lag screw, for example, has to neutralize external forces and moments to achieve stability of the fracture. Lag Screws Interfragmentary compression created by two spongy bone lag screws in the epiphyseal fracture of the distal femur. Therefore, an internal fixation of a fracture with lag screws alone is rarely stable enough to allow load bearing of the operated extremity. In most cases, a lag screw is used in combination with a plate. Compression Plate Application of interfragmentary compression by a tension device pulling on the plate. Compression hole principle Compression hole principle. When the screw is inserted into the bone, the screw head moves toward the bone, sliding on the slope and pushing the plate in horizontal direction (Δ axial). Compression holes can be used to close fracture gaps and create interfragmentary compression. Interfragmentary Compression by Tension Band Principle The application of the tension band principle by a plate fixed on the tensile force site of a fractured bone. Tension band systems Tension band principle applied for the cerclage fixation of a patella fracture. Key Point The fracture type The fracture position The loading direction Wolf’s law Biomechanics of Fracture Healing Bone Healing under Interfragmentary Movement Fracture Healing under Interfragmentary Compression Delayed Healing and Nonhealing under Unstable Fixation Bone Healing under Interfragmentary Movement Fracture healing under interfragmentary movement occurs by callus formation that mechanically unites the bony fragments. After trauma and fracture, a hematoma occurs that undergoes tissue differentiation. The sequence of fracture healing Inflammation Soft callus Hard callus Remodeling Stages in the Healing of a Bone Fracture Hematoma formation Torn blood vessels hemorrhage A mass of clotted blood (hematoma) forms at the fracture site Site becomes swollen, painful, and inflamed Stages in the Healing of a Bone Fracture Fibrocartilaginous callus forms Granulation tissue (soft callus) forms a few days after the fracture Capillaries grow into the tissue and phagocytic cells begin cleaning debris Stages in the Healing of a Bone Fracture Bony callus formation New bone trabeculae appear in the fibrocartilaginous callus Fibrocartilaginous callus converts into a bony (hard) callus Bone callus begins 3-4 weeks after injury, and continues until firm union is formed 2-3 months later Stages in the Healing of a Bone Fracture Bone remodeling Excess material on the bone shaft exterior and in the medullary canal is removed Compact bone is laid down to reconstruct shaft walls Schematic drawing of the callus healing process. Early intramembranous bone formation (a), growing callus volume and diameter mainly by enchondral ossification (b), and bridging of the fragments (c). Figure from Brighton, et al, JBJS-A, 1991. A: Roentgenogram of a callus healing in a sheep tibia with the osteotomy line still visible (6 weeks p.o.). B: Histological picture of a sheep tibia osteotomy (fracture model) after bone bridging by external and intramedullary callus formation. A few areas of fibrocartilage remain at the level of the former fracture line (dark areas). Bone Healing under Interfragmentary Movement(cont.) The flexural and torsional rigidity of a fracture depends on the material properties and the second moment of inertia (rigidity = EI) of the callus. Particularly the increase in callus diameter has a significant effect on the stabilization of the fracture. Linear relation to the mechanical quality of the callus tissue (E), The rigidity is proportional to the fourth power of the diameter (IBending = πd4/64, ITorsion = π d4/32) Bone Healing under Interfragmentary Movement(cont.) The interfragmentary movement under external loading decreases with healing time in relation to the rigidity of the callus. Finally, the hard callus bridges the bony fragments and reduces the interfragmentary movement to such a low level that a healing of the fracture in the cortex can take place (Fig). When this has happened, the callus tissue is no longer required and is resorbed by osteoclasts. Finally, after a remodeling process, the shape and strength of the normal bone are reconstituted. Healed osteotomy of a sheep metatarsal with bony bridging of the cortical osteotomy gap and only small remaining callus volume. Fracture Healing under Interfragmentary Compression implants and external loads Compression forces compression and close contact ( the external traction forces => the internal compression preload ) compression preload +friction between the fragmentsrelative movement between the fragments is avoided. Under this absolutely stable fixation, bone healing can occur by direct osteonal bridging of the fracture line with minimally or no callus formation . In areas with direct contact, remodeling starts a few weeks after fracture fixation, which leads to bridging of the fragments by newly formed osteons . Fracture Healing under Interfragmentary Compression(cont.) Haversian osteons with osteoclasts in their cutter heads resorb bone, create a tunnel that crosses the fracture line, and fill the tunnel with new bone in a process of osteoblastic activity. In areas with a gap between the fragments, a filling of the gap by woven bone occurs as a first step before the Haversian osteons can cross the fracture area . In reality a mixture of contact and gap healing will occur. Osteon with bone-resorbing osteoclasts (left) that drill a tunnel into the bone and osteoblasts that lay down new bone (osteoid) and fill the tunnel with a new bone layer (original magnification 100×) Osteoclas t Osteoblast New bone Contact healing with osteons crossing the fracture line (left). Healing of a fracture gap (right). Woven bone fills the gap before the osteons can bridge the fracture area. Fracture Healing under Interfragmentary Compression(cont.) An advantage of absolute stability is that the blood vessels may cross the fracture site more easily and lead to faster revascularization . In contrast to callus healing, there is no increased bone diameter under direct osteonal healing. This limits the load-bearing capacity of the healing bone, which consequently requires a longer period of protection by the implant. Delayed Healing and Nonhealing under Unstable Fixation When the interfragmentary movement is too large, the bony bridging of the fragments is delayed or even prevented. Large interfragmentary movements cause large tissue strains and hydrostatic pressures in the fracture that prevent the vascularization of the fracture zone. Without this vascularization bone cells cannot survive, bone cannot be built, and only fibrocartilage can be formed . Because the resisting fibrocartilage layer in between the two bony fragments looks like the image of a joint, the nonunion is also called pseudarthrosis (false joint). MAIN FACTORS INFLUENCING THE BIOMECHANICS OF FRACTURE HEALING Inter Fragmentary Movement-Axial Movement Small interfragmentary(IFM) movements stimulate callus formation Small fracture gaps Cyclic axial movement stimulated callus volume Large fracture gaps Callus formation seems to be limited and bridging of the fracture gap is delayed A more stable fixation with smaller interfragmentary movements seems to be advantageous Axial Movement Very stiff fixation of a fracture can suppress the callus formation and delay healing. In such cases, an externally applied interfragmentary movement can be used to stimulate callus healing. -- Kenwright J, Goodship AE. Controlled mechanical stimulation in the treatment of tibial fractures. Clin Orthop 1989:36-47. However, when the fracture fixation itself allows axial movements to a sufficient extent to stimulate callus formation, an additional external application of interfragmentary movements does not lead to further improvement of the healing process. --Augat P, Merk J, Wolf S, et al. Mechanical stimulation by external application of cyclic tensile strains does not effectively enhance bone healing. J Orthop Trauma 2001;15:54-60. Shear Movement Shear movement delays the fracture healing ? Impede vascularization and promote fibrous tissue differentiation Oblique tibial fracture (shear movements : 4 mm) -- treated with functional bracing show rapid natural healing Shear movement to induce delayed unions and nonunions ? Control • Shear movement • Osteotomies of oblique or transverse type Comparison Timing, magnitude, and/or gap size Tibial osteotomy in sheep 3-mm osteotomy gap Give axial or plane shear movement of 1.5 mm (Augat et al., 2003) Loading of the tibia during gait Tibial osteotomy 2.4-mm osteotomy gap 25% axial compression (0.6 mm) Torsional shear (7.2°) A displacement-controlled hydraulic actuator (Bishop et al., 2003) Blood Supply Sufficient blood supply Nutrition of the healing zone A delayed union or even an atrophic nonunion Trauma or smoking Under unstable fixation Capillaries required for osseous repair are constantly ruptured fibrocartilaginous tissue Large interfragmentary movement • Non-ossified callus tissue • Tissue strains prevent revascularization • Hydrostatic pressure a collapse of the blood vessels Biomechanical Monitoring of Fracture Healing The interfragmentary movement can be used for the monitoring of the bone healing process for patients with fracture treatment by external fixation As the healing process progresses, the callus increases in size and rigidity and shares more and more of the external load The load at the external fixator decreases, which leads to decreasing deformation of the fixation frame Therefore, the measurement of fixator deformation allows an indirect determination of the interfragmentary movement and stiffness of the callus Mechanobiology of Fracture Healing Mechanical Stimulation of Fracture Callus Cells Mechanoregulation of Fracture Healing Mechanoregulation of Tissue Differentiation Mechanoregulation Models of Fracture Healing Mechanical Stimulation of Fracture Callus Cells Fracture healing Early phase of fibroplastic stage The progenitor cells are believed to arise from the periosteum, endosteum, marrow, and surrounding extracortical soft tissue Multipotential progenitor cells begin to invade the granulation tissue callus Differentiate into various cell phenotypes and proliferate within the callus Fracture healing Early phase of fibroplastic stage away from the fracture gap and along the periosteum and endosteum, the cells differentiate into osteoblasts and begin to directly produce bone within the callus and the gap, the progenitor cells differentiate into fibroblasts or chondrocytes, proliferate, and begin to produce a fibrous connective tissue or cartilage matrix, respectively this soft tissue bridges the fragment ends and stabilizes the fracture to some degree Contemporary oral and maxillofacial surgery 5e Fracture healing Late phase of fibroplastic stage chondrocytes at the hard and soft tissue interface proliferate, hypertrophy, and calcify, forming bone fibroblasts and connective tissue are slowly replaced by chondrocytes and cartilage creeping substitution of bone from the distal ends of the callus until an initial osseous bridge of the fracture gap When the gap is filled with woven bone, the fracture is considered healed Contemporary oral and maxillofacial surgery 5e Final remodeling stage osteoclasts begin to resorb the woven bone in the extraperiosteal callus as osteonal remodeling occurs across the gap Contemporary oral and maxillofacial surgery 5e Mechanical Stimulation of Fracture Callus Cells Various cells found in the callus are modulated by the local mechanical environment Cyclic hydrostatic pressure applied to in vitro cell cultures of bone marrow-derived mesenchymal stem cells were found to enhance differentiation into chondrocytes and stimulated cartilaginous matrix Angele P, Yoo JU, Smith C, et al production Mechanical compression was also found to regulate synthesis of distinct proteoglycan types by fibroblasts in tendon explants Koob TJ, Clark PE, Hernandez DJ, et al. Mechanical Stimulation of Fracture Callus Cells When intermittent hydrostatic pressure was applied to embryonic bone organ cultures, hypertrophy of chondrocytes and mineralization were accelerated van't Veen SJ, Hagen JW, van Ginkel FC, et al. Osteoblasts have also been demonstrated to be sensitive to mechanical stimuli. Cyclic tensile strain has been found to increase their proliferation and osteoid production Kaspar D, Seidl W, Neidlinger-Wilke C, et al. In contrast, biaxial stretch was found to regulate apoptosis and proliferation of osteoblasts in a differential fashion dependent on their state of differentiation Weyts FA, Bosmans B, Niesing R, et al. Mechanical Stimulation of Fracture Callus Cells Mechanical loading applied to the callus tissue produces local biophysical stimuli sensed by the cells regulate cell phenotype, proliferation/apoptosis, and anabolic and catabolic synthesis activities with alteration of the extracellular matrix and the associated changes in material properties of the tissue Mechanical Stimulation of Fracture Callus Cells In normal fracture healing this feedback process reaches steady state when the callus has ossified and the original cortex has regenerated However, this feedback process may also explain some complications of fracture healing such as delayed or nonunions where the tissue properties combined with loading may promote the persistence of soft tissues Thus, the mechanobiology of callus cells is integral to understanding the biomechanics of fracture healing. Mechanoregulation of Fracture Healing Mechanoregulation of Tissue Differentiation Mechanoregulation Models of Fracture Healing Mechanoregulation of Tissue Differentiation Late 1800s ,Roux introduced his theory of functional adaptation He proposed that the mechanical environment or “irritations” actually stimulated the formation of particular types of connective tissue Compression stimulated the formation of bone Tension for connective tissue In combination with compression or tension for cartilage Mechanoregulation of Tissue Differentiation Almost a century later, Pauwels proposed a more rigorous mechanoregulation theory based on continuum mechanics He analyzed the mechanical environment with a healing fracture callus and hypothesized that the invariants of the strain and stress tensors guided the differentiation pathway Mechanoregulation of Tissue Differentiation Perren and Cordey believed that tissue differentiation was a result of tissue disruption if stresses exceeded the tissue strength or tissue elongation resulted in rupturing, the tissue would change its phenotype such that tissue failure would not occur using finite-element analysis (FEA) to calculate the complex tissue strain in the callus at the beginning of healing Mechanoregulation of Tissue Differentiation Cheal et al. compared histology of the fracture callus with magnitudes of strain Although they did not demonstrate tissue damage, they found an association of high strain levels with soft tissues and bone resorption low strain levels with bone formation Mechanoregulation of Tissue Differentiation Carter et al. proposed local stress or strain history as a method to allow a range of cyclically applied loads to influence tissue differentiation over time Mechanoregulation of Tissue Differentiation Claes and Heigele was initially presented in quantitative terms Mechanoregulation of Tissue Differentiation Finally, Prendergast, Huiskes, and colleagues have developed a different mechanoregulation concept taking into consideration that connective tissues are poroelastic and comprise both fluid and solid Mechanoregulation of Tissue Differentiation They all propose that higher magnitudes of tissue deformation result in the stimulation of softer fibrous connective tissue cartilage and bone are formed in the presence of lower strains Mechanoregulation Models of Fracture Healing The models are generally divided into two parts In one part, the tissue deformations and stresses are calculated using FEM of the healing fracture with tissue morphology, material properties, and loading conditions as the input Mechanoregulation Models of Fracture Healing In the other part, the mechanoregulation algorithm is described by a set of mathematical or logical rules and used to predict changes in tissue material properties Mechanoregulation Models of Fracture Healing Now that these complex models have been developed The next challenge will be to compare them with known in vivo results demonstrating the mechanosensitivity of tissue differentiation during fracture healing With such comparisons, the most significant mechanobiological interaction can be resolved and mechanically guided tissue transformation functions defined These could then be combined with dramatically improving computer and imaging technology (computed tomography, nuclear magnetic resonance) and musculoskeletal loading simulations to develop fracture healing models that would enable us to optimize fracture treatment for individual patients from a biomechanical point of view Thanks for your attention!