Rethinking FDA`s Food and Supplement Framework:
advertisement

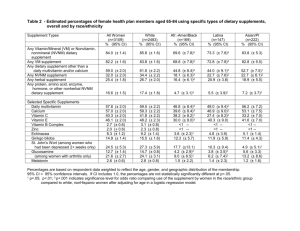
Rethinking FDA’s Food and Supplement Framework: Does the Food vs. Supplement Distinction Still Make Sense? Michael Gruver Kaye Scholer LLP July 22, 2014 Topics to Cover • Background on January 2014 Guidance for Liquid Dietary Supplements • Impact of the Food vs. Supplement Distinction • January 2014 Guidance in Detail • Analysis & Policy Questions 2 Introduction • In January 2014, FDA released a guidance for industry: “Distinguishing Liquid Dietary Supplements from Beverages.” – Expanded version of 2009 preliminary guidance. – Concerned about an “increase in the marketing of liquid products with a wide array of ingredients and intended uses.” – Guidance issued to “help dietary supplement and beverage manufacturers and distributors determine whether a product in liquid form is properly classified as a dietary supplement or as a beverage.” – 8 factor test for determining food or supplement. • Growing sensitivity at FDA regarding beverage products potentially misbranded as dietary supplements. 3 Food vs. Supplement Basics • Beverages are considered traditional foods under the FDCA. – “[U]sed for food or drink for man or other animals.” 21 U.S.C. §321(f)(1). • Dietary supplement contains either specific category of ingredients (e.g. vitamins) or other “dietary substance for use by man to supplement diet.” – A product “not represented for use as a conventional food or as a sole item of a meal or the diet.” 21 U.S.C. §321(ff). • Beverages serve the basic purposes of the human diet. – Nutrition, Hydration, and Taste. • Supplements are a compliment to the nutrition from foods and beverages. 4 Importance of the Distinction • Supplements considered more “drug like’ -- Must include disclaimer saying not intended to treat, diagnose, or cure disease. – No supplement may be marketed if it was the subject of a substantial clinical development program aimed at drug approval. – Manufacturers of supplements must report adverse events. • Different labeling requirements. – “Supplement Facts” vs. “Nutrition Facts” • Treated differently with respect to GRAS criteria. – Food additives require premarket approval unless GRAS. – New Dietary Ingredients require NDI notification to FDA, but not required to be GRAS for their intended use in a supplement (e.g. caffeine). • Non-dietary ingredients must be approved or GRAS (e.g. binders) 5 Overview of New Guidance • 8 factors determine whether a liquid product can properly claim to be a beverage or a dietary supplement. • Answer depends on the overall balance of the factors. – No guidance as to how factors should be balanced or would be interpreted by the FDA or a court. • Market-Drive Approach: – Heavy focus on how a product is marketed, labeled, or positioned in the marketplace. – Composition appears a secondary consideration. – Ask: Is the product advertised as providing some form of nutrition on its own, or does it merely claim to enhance diet? 6 Labeling and Advertising Claims • Key inquiry is what benefit the product claims to provide. • Liquid product that claims to “refresh” or “rehydrate” would probably qualify as a food even if no other nutritional value. – Can also include visuals (e.g. supplement salad dressing) • May turn on comparison to other products. – e.g. “Tastes better than Pepsi or Coke.” 7 Representations Outside of Labeling and Advertising • Claims made about a product in publicly available documents other than advertising. – Patent and Trademark Office – Securities and Exchange Commission • Likely far less important in the total calculus of factors than claims directed at a broader audience. • Still, relevant (e.g. marketed as a beverage while patented as a nutritional supplement). 8 Product Name • Factor most easily used to steer a product toward one classification. • Brand names which use conventional food terms such as “beverage” “drink” “water” or “soda” represent the product as a beverage. • FDA attaches great significance to a liquid product’s name. – “In some instances, the mere use of such a term in a product name or brand name may be sufficient to establish that the product is represented for use as a conventional food.” Guidance at p. 3 (January 2014). • Consider energy “drinks” marketed as supplements. 9 Packaging • Must consider: – – – – – Size Shape Color Volume Resealable vs. Single-Serving • Similarity to other types of packaging is also important. – A liquid product packaged “in a red, 12 ounce pop-top aluminum can bearing a silver stripe with the name ‘Cola Supplement’ printed on the can” is likely a beverage. Guidance at p. 3 (January 2014). 10 Serving Size and Recommended Daily Intake • Relies on survey data that the average American adult consumes 1.2 liters of fluids other than water in a day. • Liquid products that suggest consumption in amounts approaching 1.2 liters present themselves as beverages. – Beverages are products intended to constitute the source of that 1.2 liters. (e.g. “Drink up to three 16-ounce bottles per day.)” • Liquids that suggest consumption at far lower levels present themselves as supplements. (e.g. “energy shots”) 11 Recommendations and Directions for Use • Supplements – Product should be used in conjunction with conventional food or drink. • Beverages – Quench thirst or provide a source of fluids (e.g. water). – Provide nutritive value (e.g. juice or milk). – Taste (soda). • Example: Liquid Vitamin C – Recommend as a better way to obtain Vitamin C = Supplement. – Mention that it can rehydrate or tastes good = Beverage. 12 Marketing Practices • Focus on ways of positioning a product in the marketplace, such as sponsorships, product placement and meta-tagging. – Social Media Practices: In 2012, FDA issued a warning letter to a drug-maker for “liking” an unapproved claim on facebook. • Strong emphasis on whether and how product is compared to “traditional” forms of beverages or supplements. – Marketing practices traditionally associated with products in one category? – Including location of product in stores. • Broadest factor may be the most confounding. – Consider new supplement sold in dedicated cooler units. 13 Composition • Lone factor that deals with the actual make-up of the product. – No clarity as to how it weighs against the other seven factors. • Undeniable areas of overlap in terms of the ingredients in foods and supplements. – FDA maintains that the overlap “is not intended to be total...[it] would strain common sense to authoriz[e] the creation of a dietary supplement whenever any dietary ingredient is added to a conventional food.” See Guidance at p. 4 (January 2014). • Concern that, without considering composition, manufacturers might attempt to evade GRAS requirements simply by adding inconsequential dietary ingredient. • Mindful that certain dietary ingredients that may lawfully be added to supplements may not be lawfully added to foods. (e.g. ginko biloba) – Certain color additives may also be used in supplements but not in foods. 14 How You Sell it Matters More than What You Sell? • Far more questions than answers: – What concentration of dietary ingredients in the overall composition of a product qualifies that product as a supplement? – Is the relative concentration of dietary ingredients more or less important than the manner in which dietary ingredients are emphasized in packaging or marketing? – If how a product is marketed or advertised outweighs the product’s actual composition, why consider composition at all? – Are there products that would qualify as foods or supplements no matter what their packaging says or how the product is marketed and promoted? 15 Not Much Guidance • Basing guidance in marketing/advertising makes it elastic. • Factual scenarios that can change quickly. • Governed by how the product compares to other “traditional” products. – Package Size – Labeling – Placement in Store • Beverage is one advertising claim or store shelf away from becoming a supplement, and vice versa. 16 Recipe for Confusion • Limited utility to seeking advice from the FDA prior to introducing or altering product, packaging, or marketing. – Business-driven change to any factors might alter balance of FDA analysis. • In current food/supplement litigation environment a single word or phrase can give rise to class action claims. – Accusation that product is “falsely” marketed or sold. – Little guidance to Courts in determining the merits of those claims. – Likely to see conflicting rulings based on varying interpretations of the FDA’s stated criteria. 17 Does Separate Treatment Continue to Make Sense? • “Foods” can become “supplements” based on how they are packaged, labeled, marketed or placed within a store. – Questionable benefit to maintaining ephemeral distinction. • Significant cross-over of ingredients between two classes of product. – FSMA revising “Nutrition Facts” to increase clarity of information. – Questionable to maintain separate rules for “Supplement Facts.” • Wavering distinction brings significant risk for manufacturers. – Every misapplication of the food/supplement distinction yields a misbranded product. • Lack of clarity of no benefit to consumers. 18 Advantages of Single Standard • A single uniform standard would give consumers clarity with respect to the nutritional content of what they purchase or consume. • Provide suppliers with a clear set of guidelines. – Based on content as opposed to subjective interpretation of marketing intent. • Encourages consistent representations in all venues. • If distinction must be maintained, it should be meaningful. 19 Policy Proposals • Develop a comprehensive and uniform set of guidelines for both foods and supplements. – e.g. Single ingredient disclosure scheme. • If distinction is maintained, should at least: – Provide distinct definitions to determine whether a product is a food or a supplement.. • Avoid defining products solely in relation to each other. – Emphasis on concrete factors such as composition or recommended daily intake. – Require “Fact” panels to provide the same categories of information. • Facilitate customers’ ability to determine the differences between individual products. – Reduce emphasis on elastic factors such as marketing strategy or product placement. 20