Ian Davis presentation

2011 ASCO Genitourinary
Cancers Symposium
17-19 February 2011, Orlando http://2011.gucasym.org/ www.anzup.org.au
General
• Approximately 500 participants, seemed more
• Program:
• Day 1: Prostate
– General Session I: Emerging Trends in the Characterization and Treatment Decisions in
Newly Diagnosed Prostate Cancer
– General Session II: Prostate Cancer Therapy for Recurrent Disease
– General Session III: Translational Science Session: New Targets for Prostate Cancer
Therapy
• Day 2: urothelial, penile, urethral, testicular
– General Session IV: Urothelial Carcinomas: Cases in Perioperative Chemotherapy
– Keynote Lecture:
• Stem Cells and Tumorigenesis in Genitourinary Tumors. Carlos Cordon-Cardo, MD, PhD -
Columbia University Medical Center
– General Session V: Penile, Urethral, and Testicular Cancers
– General Session VI: Translational Science Session: Urothelial Carcinomas
• Day 3: Renal
– General Session VII: Renal Cancer
– General Session VIII: Translational Science Session: Renal Cancer^
• Interspersed poster and poster discussion sessions, ticketed sessions
• Special seminars: emphasis on prostate cancer
Luo J, Solimini NL, Elledge SJ: Principles of Cancer Therapy: Oncogene and Non-oncogene Addiction. Cell 136:823-837, 2009
High points: prostate
• PSA and GS are now included in AJCC stage
• NCCN now has “very low” risk category
– T1a: GS<6 <3 cores +ve and <50% involved
• New pathology reporting standards (next slide)
• PSA doubling time after RP:
– <4 months: probably metastatic
– >12 months: more likely local recurrence
• In testing for micrometastatic disease:
– RT-PCR
– CTC cut point 5 / 7.5 mL
– CTC-chip
– Circulating exosomes
• Three new treatments approved in US in 2010 for CRPC:
– Cabazitaxel, sipuleucel-T, denosumab
– (Abiraterone approved April 2011)
De Margo (Johns Hopkins): pathology
• TRUS is insensitive
– ~20% of patients are upgraded at RP
– One biopsy core ~ 1/3000 weight of prostate
• New markers:
– 34βE12 and p63 = basal markers; absent in PC
– AMACR positive in PC
• New pathology reporting:
– Always report secondary pattern of higher grade if present even if minor component eg 5%
– Separate GS report by core
– On core biopsy: any Gleason 5 implies high grade, called 8-10
– At RP: report primary/secondary and comment on tertiary
– Ductal adeno: automatically called 4+4=8
– Cribriform pattern previously 3 now 4
Shipley (MGH): RTOG 9601
• Background and rationale: to determine if long term antiandrogen therapy with RT improves cancer control and OS
• Design:
– Phase III, double-blind, placebo-controlled
– Postoperative pT2-3, N0, positive margins, elevated PSA <4 postop, negative scans
– RT ( RT (64.8 Gy in 1.8 Gy fractions) ± bicalutamide 150 mg/d during and after RT x 24 months
•
Note: not current treatment regimen
– Stratification: margins; nadir PSA < 0.5; entry PSA < 1.5; neoadjuvant short term ADT
– Primary endpoint: OS
•
Demographics:
– 771 patients, median age 65
– Median 2.1 yr between RP and study entry
– Median time RT to positive PSA 1.2 year
•
PSA failure defined as 0.4 from undetectable, or increase 0.3 above entry PSA
•
Results:
– 1-3% gr 3 early GU toxicity, 6% late
– 0.3-1% early gr 3 GI toxicity, 2% late
– OS 91% vs 86%; too few events yet (primary endpoint) B vs plac
– Mets: 7.4 vs 12% (p<0.041)
– FFP at 7 years: 57 vs 40% (p<0.02)
– Benefit across all groups
– PSADT benefit except in >2yr group
– Gynecomastia led to withdrawal in 8%
Fleshner (Toronto): REDEEM
• Reduction by Dutasteride of Clinical Progression Events in Expectant
Management of Prostate Cancer
• Testing whether dutasteride controls growth of existing low risk, localised prostate cancer reduces need for aggressive therapy in men followed with active surveillance
• 302 men, aged 4882, PSA <11 ng/mL, and GS ≤6 PCa (≥10 cores, ≤3 cores positive, <50% of any core positive)
• Randomised to dutasteride 0.5 mg/d or placebo for 3 years
• Repeat 12-core biopsies at 18 and 36 months, or for-cause at other times during the study
• Primary endpoint TTP: time to therapy, or pathology with ≥4 cores positive, or ≥50% involved or any GS ≥4
• Results:
– HR 0.61 risk of progression
– No cancer found in 23% of placebo and 36% dutasteride at 36 months
– QoL: less anxiety and fear of recurrence in D group, perhaps due to information about PSA
– No effect on sexual function
– No evidence of increased Gleason score upgrading with dutasteride
• Note: D shrinks gland so more likely to find any residual cancer
• Note: FDA warning issued 9 June 2011
Androgen Resistance:
Overlapping mechanisms
LHRH
Novel
Antiandrogens
AR amplification
(30%)
AR mutations?
CRPC
Intratumoral androgen production/conversion
Finasteride/Dutasteride
Ketoconazole
Abiraterone
Antiandrogens
Other proposed (outlaw) pathways:
• Indirect (ligand-independent) activation of AR activated in absence of androgen
•
Via tyrosine kinases (epidermal growth factor receptor), cytokines (interleukins)
• Signal transduction pathways nuclear factorκB
•
Apoptotic pathways
Persistent serum androgens (eg, adrenals)
Ketoconazole
Abiraterone
Steroids
Antiandrogens
Modified from: Van Allen EM, Ryan CJ. Curr Opinion Urol . 19:315-321. Bonkoff H, Berges R. Prostate.
2010;70:100-112.
Multiple mechanisms of action: points of targeted intervention in AR pathways
2.
Abiraterone ketoconazole
1. 17-AAG
AR degraded
Androgen precursors
AR
HSP90
2.
Adrenal synthesis
Tumor synthesis
Abiraterone ketoconazole
Androgens
Cell surface ligand/receptor
5. TKI inhibitors, antibodies
3. 5
-reductase
DHT inhibitors
Ack1 SRC mut
AR
AR AR
4. MDV-3100
BMS641988
P
AR AR
P
5. Dasatinib
Amp
AR
Antiandrogens, progestins, glucocorticoids
4.
MDV-3100
BMS641988
P
AR AR
6. HDACi (SAHA, LBH589)
P
Transcription of TMPRSS-ETS, etc for growth and survival
From: Chen Y et al. Curr Opin Pharmacol.
2008;8:440-448.
Study 301 Phase 3:
Randomized, Double-Blind,
Placebo-Controlled Trial in Patients
With CRPC Who Have Failed
Docetaxel-Based Chemotherapy
Prior Chemotherapy
Prednisone Add-on Therapy
Clinicaltrials.gov identifier: NCT00638690.
COU-AA-301 study design
•
Phase 3, multinational, multicenter, randomized, double-blind, placebocontrolled study (147 sites in 13 countries; USA, Europe, Australia, Canada)
•
Primary end point:
– 25% improvement in overall survival (HR = 0.8)
•
Secondary end points:
– Proportion of patients achieving a PSA decline ≥ 50% according to PSAWG criteria; time to PSA progression according to PSAWG criteria; PFS based on imaging studies;
CTC counts and profiling with outcome
• Stratification according to:
– ECOG performance status (0-1 vs. 2)
– Worst pain over previous 24 hours (BPI short form; 0-3 [absent] vs. 4-10 [present])
– Prior chemotherapy (1 vs. 2)
– Type of progression (PSA only vs. radiographic progression with or without PSA progression)
•
Data presented from interim analysis
Clinicaltrials.gov identifier: NCT00638690
COU-AA-301: All Secondary End
Points Achieved Statistical Significance
TTPP (months)
AA
(n = 797)
10.2
Placebo
(n = 398)
6.6
3.6
HR
95% CI
0.58
(0.46, 0.73)
0.67
(0.59, 0.78)
P Value
< 0.0001
< 0.0001
rPFS (months) 5.6
PSA response rate
Total
Confirmed
38.0%
29.1%
10.1%
5.5%
< 0.0001
< 0.0001
COU-AA-301: Abiraterone Acetate
Improves Overall Survival in mCRPC
HR = 0.646 (0.54-0.77) P < 0.0001
100
Abiraterone acetate:
14.8 months (95%CI: 14.1, 15.4)
80
60
AA
Placebo
40
20
Placebo:
10.9 months (95%CI: 10.2, 12.0)
2 Prior Chemo OS:
14.0 mos AA vs 10.3 mos placebo
1 Prior Chemo OS
15.4 mos AA vs 11.5 mos placebo
0
797
398
100
728
352
200
631
296
300 400 500
Days from Randomization
475
180
204
69
25
8
600
0
1
700
COU-AA-301 Conclusions
AA prolongs OS in patients with mCRPC who have progressed after docetaxel-based chemotherapy
• AA plus prednisone significantly improves TTPP, rPFS, and PSA response rate
– 35% risk reduction of death (HR = 0.65)
– Median OS improvement with AA of 14.8 vs 10.9 months with placebo
– 36% improvement in median OS
– For patients with 1 prior chemo regimen
• Median OS improvement with AA of 15.4 vs 11.5 months with placebo (HR = 0.63)
– Median time to PSA progression and median time to rPFS significantly improved
OS Benefit in Recent CRPC Trials
Trial/
Agent Approved
IMPACT
(Provenge vaccine)
2010
TAX327
(Docetaxel)
2004
TROPIC
(Cabazitaxel)
2010
COU-AA-301
(Abiraterone acetate) 2010
Disease state
Chemonäive
CRPC
Chemo-
CRPC näive
Post-
Docetaxel
CRPC
Post-
Docetaxel
CRPC
Comparator
Placebo
Mitoxantrone
Prednisone
Mitoxantrone
Prednisone
Placebo
Prednisone
Hazard
Ratio
0.775
P value
0.032
0.76
0.70
0.646
0.009
<0.0001
<0.0001
Other prostate highlights
• Roach (UCSF): management of radiation failures
– Various patterns but often isolated local recurrence
– Salvage RT safe and effective
• Scardino: fewer mets with RP than RT. Note: RP→RT salvage 56%; RT→RP salvage 2% AND earlier: 13 months vs 69 months
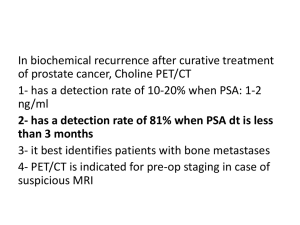
• Barentsz: imaging
– DCE MRI 92% sensitivity, 85% specificity, NPV 95%, PPV 46% needing biopsy
– 77% of recurrences are in nodes not in CTV
– DWI MRI resolution 5mm, measures restriction of water flow ie nodes look black
– 11 C-choline: resolution ~ 5mm
• Small (UCSF):
– PSADT predicts mortality after RT
Prostate (cont)
•
Bul (Rotterdam): ERSPC
– 162,387 men, 4-yearly screen, control = standard of care
– PSA cut off = 3.0 then biopsy
– Endpoint: PC mortality
• 9-year followup: mortality decreased 30%
• Rotterdam cohort:
– 42,376 men
– 19,950 screened first round, 15 year followup
– 15,758 initial PSA <3
– 915 = 8.5% PC
– 23 deaths: 5 screen detected, 18 interval
– Mortality increases with higher PSA
• Klotz (Toronto): IADT vs continuous ADT for PSA progression after definitive therapy
– NCIC PR.7: premature stop at interim analysis
– 1386 randomised, 690 IADT, 696 continuous
– IADT: 37.6 no-treatment months, 15.4 on treatment ie 27% on treatment
– OS identical HR 1.02, non-inferiority p 0.009
– Median survival 9 years
– Time to CRPC median 10 years (?)
– Stratify by log rand p=0.024 in favour of IADT but design bias (had to rechallenge in that arm to prove refractory)
– Mortality 18 vs 15%, HR 1.18 p 0.24
– AEs similar re worst events, relate to on-treatment time
– Off treatment QoL data not yet available
Bladder/urothelial
• Gallagher (Dublin): SNPs and chemo sensitivity
– More responses to cisplatin than carboplatin
– MSKCC risk factors: visceral mets Y/N; KPS <80 vs ≥80
• 0: median survival 33 months
• 1: 13 months
• 2: 9 months
– 4 SNPs identified, each scored 0-2 based on allele presence
– Score 0: 80% response; score 8: <30%
– Genes: IL-1β, CCND1 (cyclin D1), PARD6B (cell-cell interaction, insulin signalling), Rs1520896 (chrom 11, gene unknown)
• Case studies:
• “Mixed histology might respond better to MVAC” – not in MDACC data
• GC as neoadjuvant treatment?
• Dose dense MVAC effective (Sternberg)
• GC 7% pathological CR compared to 20-40% MVAC
• RT less effective in extensive CIS
• MMC + 5FU + RT tested in patients with GFR >25 mL/min (James –
UK)
Other bladder/urothelial clinical highlights
• Galsky: cisplatin-ineligible TCC:
• Proposed any of:
–
PS2 (KPS 60-
70); CrCl <60; CTCAE v4 ≥2 hearing loss or neuropathy; NYHA class III heart failure
• Morales: cisplatin-ineligible TCC:
–
Gemcitabine 2500 mg/m 2 on day 1 and cisplatin 35 mg/m 2 on day 1, every 14 days. 40 patients, 36 evaluable for response.
– Mean creatinine clearance was 49 mL/min (range:37-59 ml/min)
– Well tolerated overall
–
One complete response, 14 partial responses (ORR: 42%; 95% CI 27-58%), 11 stable and 10 PD
– Median progression-free survival 15 weeks
–
Median OS 35 weeks and 1-year OS is 43%
• Other regimens:
• Pagliaro: gemcitabine / paclitaxel / doxorubicin for urothelial cancer and CrCl <60
– G 900 mg/m 2 , P 135, D 40, on d1 q2wk with peg-filgrastim
– 27 pt; 23 assessable for response
• 4 CR and 9 PR = RR 56.5%
• Median OS 13.8 months
• Sridhar: Nab-paclitaxel: 32% PR 53% median PFS 6 months, OS 10.8.
– Increased survival if Hb>100, PS≤1, chemo >5 months ago, get disease control
• Wong: cetuximab/paclitaxel
– C inactive as single agent
– Combo RR 25% with 1 CR and 6 PR in 28 pt
• Smith: carbo/GCB/ABI-007 (nab-pac)
– pCR 27% and get <T2 in 54% ie only non-invasive cancer left
Germ cell
• Not much
• Huddart (Singhera): late CT surveillance in NSGCT
– Relapse ~3% in literature
– Usually metastatic NSGCT, increased AFP, relapses between visits
– Usually require surgery (resect >1cm post chemo)
• Einhorn (Indiana): residual masses
– Do PET wk 6 post chemo: should be negative (SUV <4)
– If positive: consider resection BUT strong desmoplastic reaction after chemo for seminoma
– HR 1.3 for second cancers after RT for seminoma
– 0.4-0.9% leukemia – bladder cancer
– NSGCT:
• They never do RPLND for <1cm nodes
– If >1cm:
• 7% cancer
• 68% teratoma even if no teratoma in primary
• 25% necrosis
– Late relapse usually found by increased AFP (not bhCG) and cured with surgery
(rare cure with chemo alone)
– “NCCN surveillance recommendations excessive”
– Indiana: CT q4mo for 2 yr then q6mo to five years
– Do abdo/pelvis only with CXR; arguably don’t do pelvis
Penile cancer
• Thankfully no horrible brachytherapy photos this year
• Pettaway (MDACC): overview
•
Bulky primary: penectomy, recurrence <10%
• Might not need 2cm margin
• High grade disease: only 25% have up to 10mm microscopic extension
• Nodes:
– 1-3: 60-80% 5 year DFS
• Controversial to dissect impalpable disease but Dutch study showed good benefit
• Imaging of LN is insensitive
• Surgery then adjuvant RT
•
Adjuvant chemo for palpable LN:
– Cisplatin/MTX/bleo or VCR/MTX/bleo
• Role for neoadjuvant chemo:
– Cisplatin – taxane/FU/irinotecan etc
– MDACC: paclitaxel/ifosfamide/cisplatin ph2 trial (Pagliaro JCO 2010)
RCC: Wood (MDACC)
• TKIs and surgery
• Various adjuvant studies: ARISER, ASSURE, S-TRAC, SORCE, pazopanib x1yr
• CARMENA French study: non-inferiority sunitinib vs surgery then sunitinib
• Neoadjuvant:
– RR <10% in primary in most series
– Response in primary usually occurs within 60 days
– If no early response there won’t be one
• Tumour thrombus: occasional response, 15% PD; most don’t respond
• (Note: poster showing that response in primary predicts outcome)
• Overall: safe (some dehiscence even late); unreliable at downstaging BUT if see early response then patients do better overall
Atkins (Boston): non-clear-cell RCC
• Papillary do reasonably well in primary but badly when metastatic
• Description of evolution of sarcomatoid RCC subcutaneous metastasis from clear cell primary resistant to sunitinib
– Transplanted into mouse, became clear cell again and restored sensitivity
• Immunotherapy inactive against nccRCC
• Collecting duct:
– Treat as TCC
• Sarcomatoid:
– GCB/doxorubicin: Hass 18% PR
– Sunitinib/sorafenib: PR 9% and only if mostly clear cell with <20% sarcomatoid
– GCB/sunitinib: 3/9 PR but no response if underlying papillary or chromophobe
• Papillary:
– RR 17% with sunitinib, 0% sorafenib
– ARCCS study: sorafenib 23% RR BUT only 3% confirmed responses
– Sunitinib (Gore): PR 11%
– TMS/IFN Dutcher paper Med Oncol 2009: 11.6 mo OS, 7.0 PFS
– HLRCC: FH mutation, increased LDH-A
– HIF-1α mediated, not HIF-2α
• BHD and chromophobe:
– Folliculin mutation
– Activation of mTOR pathway through TSC
Tumour type
Sarcomatoid
PRC1
PRC2
Chromophobe
Collecting duct
Atkins: nccRCC
Primary treatment Possible?
Gem/doxorubicin
? Met inhibitor
?VEGFR inhibitor
?mTOR inhibitor
Local therapy
Carbo/paclitaxel
Sunitinib/GCB
?TMS
?TMS
?erlotinib
LDH-A inhibitor mTOR inhibitor
Gem/cisplatin
RCC: cessation of sunitinib
• Sadegji
• Retrospective analysis: patients with SD or better and then treatment ceased for reasons other than PD
– 40 pt, all clear cell, all had nephrectomy
– Lung and LN mets commonest
– Most on first line therapy
– Time since diagnosis to treatment = 48 months – indolent group?
– Most intermediate Heng risk
– Most on sunitinib, median 14.6mo
– Most had PR, some CR
• Usually ceased because of GI symptoms, then cardiac and vascular events;
15% patient choice
• 25/40 developed PD; 15/40 still SD compared to best prior response, all still on observation
• Of the 25:
– 9/25 treated with sunitinib; 8 continued observation because low volume; 8 had local treatment (RT/surgery)
– Median followup 29.7 mo
– PFS 10 mo (1.4-27.2) in 25 pt
– 7/25 had PD at new site
• Now trial of intermittent sunitinib NCT 01158222