April 24, 2013
Naomi B Haas, MD
Associate Professor of Medicine
Abramson Cancer Center
Modulation of androgen and testosterone
New therapies for castrate resistant
prostate cancer
Intratumoral testosterone
Androgen receptor (AR) mutations and splice
variants
Ligand modulation (things that influence the
AR)
Targets in advance disease
Castrate-treated with androgen deprivation
therapy
Non-castrate- not previously treated with
androgen deprivation therapy
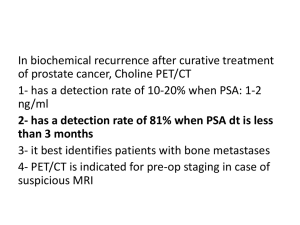
Rising PSA after surgery or radiation or both
New metastatic disease and rising PSA :noncastrate (not previously treated with
androgen deprivation therapy)
Metastatic castrate prostate cancer
Orchiectomy
LHRH (GHRH) (Luteinizing hormone releasing
hormone) agonists
Anti-androgens
Anti-androgen
Pills
LHRH
Implants and shots
LHRH antagonist- degarelix
Tiredness
Metabolic syndrome- weight gain, high blood
pressure and high blood sugar
Osteopenia-decreased bone density
Secondary risks for heart attack, blood clot or
stroke
Mood changes
Loss of sex drive (libido)
Hot flashes
Prednisone 10 mg by mouth two times a day
can decrease PSA by more than 50% in
approximately 1/3 of patients with hormonerefractory progressive prostate cancer (Sartor O
et al, The Journal of Urology Vol161, Issue 1, January 1999,
Page 360
Scholz M et al. J Urol. 2005 Jun;173(6):1947-52.
78 patients
0 1 to 3, >3 lesions bone scan
25, 35, and 18 patients
Median and mean time to PSA progression was 6.7 and 14.5 months.
Median and mean survival time was 38.0 and 42.4 months, respectively.
Response time and survival were highly correlated (r = 0.799). A total of
34 (44%) men had a greater than 75% decrease in PSA. The median
survival times in men with more vs less than a 75% decrease were 60 vs
24 months, respectively.
Lyase inhibitors- get rid of intratumoral testosterone and
residual sources of testosterone/androgens
Abiraterone acetate and prednisone
Tax 700
Toc 1 (dual lyase and AR inhibitor)
AR inhibitors- address mutations in the receptor, splice variants
MDV3100
Aragon agent
Other AR Modulators
HSP 90 inhibitors
HDAC inhibitors
Prednisone
Ketoconazole
Abiraterone
AA (Zytiga) 1000mg qd + pred 5mg twice daily
14 of 35 pts had decrease in PSA of >50%
Phase III trial completed post chemotherapy showed overall
survival improvement of almost 5 months in a study of
1000+ patients, leading to FDA approval
Dizziness
Fatigue
Low or high blood pressure
Fluid retention
Elevation of liver enzymes
Low potassium
AR modulation
1:1 randomization
Decline docetaxel
or are not suitable
for docetaxel
? patients
Coming soon
MDV3100
Something else
2:1 randomization
Failed 1 or 2 prior
chemotherapies
(docetaxel)
MDV3100
Placebo
1170 patients
Improvement in overall survival of more than 5 months
2:1 randomization
Asymptomatic
Castrate
metastatic disease
850 patients
Closed to accrual in the US
MDV3100
Placebo
ARN-509 versus MDV3100
ARN-509 versus MDV3100
Phase 1 Study Design
ARN-509
Single
Dose
Optional
FDHT-PET
at
Baseline,
4 and 12
wks
PSA
and CTC
Q 4 wks
Tumor
Evaluation
Q 12 wks
Disease
Progression
ARN-509 once daily until progression
PK week
Continuous Daily Dosing
PK D1-6
Wk 1
Cycle
2
3
4
5
1
9
2
13
3
DLT period for dose escalation
ARN-509 dose escalation cohorts (n=3-6/cohort):
30, 60, 90, 120, 180, 240, 300, 390 and 480 mg
PSA Response Rates
100
Dose
30 mg
60 mg
90 mg
120 mg
180 mg
240 mg
300 mg
390 mg
480 mg
% PSA Change from Baseline
75
50
25
0
-25
-50
-75
-100
14 out of 29 patients (48.3%)
experienced ≥ 50% reduction in PSA at 12 weeks
F-DHT-PET: Pharmacodynamic
Marker
OF AR INHIBITION IN RESPONSE TO ARN-509
Baseline
4 Weeks
Ongoing Phase 2 Trial
Non-Metastatic (M0)
CRPC
patients
(n = 93)
Metastatic
Treatment-Naïve
Metastatic
Post-Abiraterone
Primary Endpoint:
12-week PSA response
ASCO GU 2013
Provenge
Prostvac
CARs
randomized (2:1) to receive 3 doses of
sipuleucel-T (n = 341) or placebo (n = 171)
intravenously at 2-week intervals
median survival of 25.8 and 21.7 months
survival probability at 36 months of 32.1%
and 23.0% in the sipuleucel-T and placebo
arms
Kantoff GU ASCO 2010
Harness antigens expressed uniquely by a
cancer (for example Prostate specific
membrane antigen, prostate specific stem
cell antigen, F77, c-met ) and link to T cells
to turn on immunity against the antigen
ongoing trials in leukemia, pancreatic cancer
Can be given IV or into the tumor
Targets c-met and VEGFR2 both important
targets in prostate cancer
c-met is overexpressed in bone metastases
as a later event in men on androgen
deprivation therapy
VEGF expressed in aggressive prostate
cancer
RDT trial in patients previously treated with
docetaxel showed 86% had response in bone
scan; 65% had improvement in pain
Expanded prostate trial 64% (51/80 pts
evaluable) had a PR on bone scans, 24 pts
(30%) SD at 100mg daily
other cohort treated at 39 mg daily results
pending
Two new phase III trials of XL184 coming
XL 1129-2408
Screening
Week 6
Original
Normalized
CAD Annotated
Screening
Week 6
Original
Normalized
CAD Annotated
Screening
Week 6
Original
Normalized
CAD Annotated
XL 1521-2565
Screening
Week 6
Original
Normalized
CAD Annotated
Adjuvant/
Neoadjuvant
Rising PSA Only
Rising PSA
and
metastatic
disease
(noncastrate)
Progression
after ADT
(castrate)
Progression after
Docetaxel
TKIs +ADT
ADT
ADT
Provenge
Cabazetaxel
Docetaxel
ECOG 2809
ketoconazole
mitoxantrone and
prednisone
abiraterone
abiraterone
docetaxel
enzalutamide
Strive
Prevail
XL184?
Radium chloride
Biopsy with molecular profile
Treatment with chemotherapy or targeted
agents or more hormonal therapy depending
on your molecular profile
Hormone Sensitive v. Hormone Refractory Prostate Cancer
Clinical Trials
Open or Planned at
UPENN
Biology
Hormone Sensitive
Hormone Refractory
1. High risk RT+ ADT+/- docetaxel
trial
2. everolimus + salvage XRT
3. Phase I Docetaxel/ cmet
inhibitor trial
4. CAR-T cells in advanced disease
5. TKI258 plus INC280
Combines VEGFR+ FGF inhibitor with a C-met
inhibitor.
Phase I/II planned