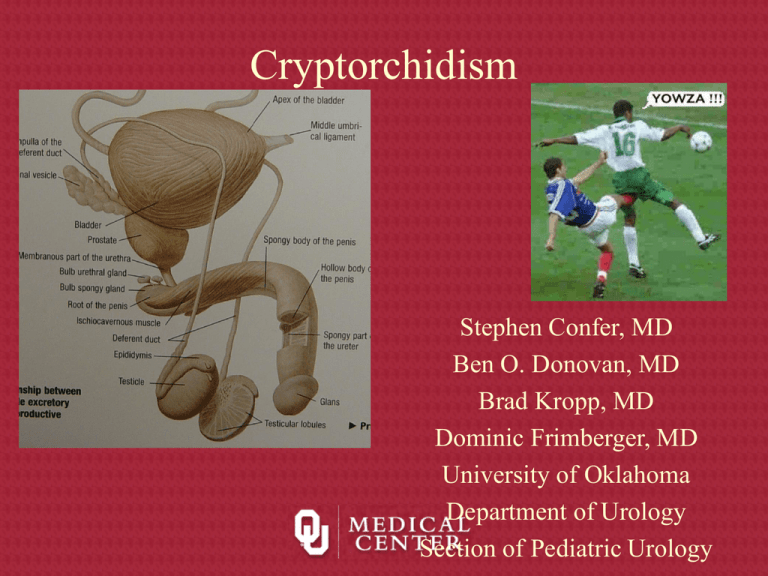
Cryptorchidism
Stephen Confer, MD
Ben O. Donovan, MD
Brad Kropp, MD
Dominic Frimberger, MD
University of Oklahoma
Department of Urology
Section of Pediatric Urology
Embryology of Gonads
• 5th week - primordial germ
cells migrate from yolk
sac along dorsal
mesentary to the posterior
body wall
Embryology of Gonads
Migration of primordial
germ cells migrate from the
posterior extraembryonic mesoderm
through the mesenteries
and into the gonadal ridge
Embryology of Gonads
• 5 weeks- Sexually indifferent –
identical structures in male and
female
• Gonadal ridges develop on
dorsal abdominal wall week 5
– future ovaries or testes
• Wolffian ducts – future male
ducts
• Müllerian ducts – future
female ducts
Embryology of Gonads
• Mesenchymal proliferation occurs and genital ridges are formed
• Paramesonephric (Mullerian) ducts begin to form lateral to the
mesonephric ducts
• After the 6th week genital ridges pursue fates as male or female.
• “Y” does this happen?
Molecular Determination of Sex
SRY acts on the indifferent gonad to
start the process of male sexual
development
Male Genital Development
• Sex cord cells differentiate into
Sertoli cells only if SRY protein
is present
• 7th week – Sertoli cells organize
to form testis cords
– Will eventually form
seminiferous tubules
– Testis cord distally forms rete
testis
– Medially rete testis connect to
mesonephric ducts via 5-12
tubules (efferent ductules)
• Vas deferens develops from
mesonephric duct
Male Genital Development
• As Sertoli cells
differentiate in response to
SRY, secrete a
glycoprotein hormone
Mullerian-inhibiting
substance (MIS)
– MIS causes
paramesonephric
(mullerian) ducts to
regress between 8th10th week
– Remnants may be
found
• Appendix testis
Male Genital• Development
9 -10 weeks – Leydig cells
th
th
differentiate
– Early testosterone regulated by
placental chorionic gonadotrophin –
eventually regulated by pituitary
gonadotrophins
• 8th-12th weeks – testosterone
secretion by Leydig cells stimulates
transformation of mesonephric duct
to the vas deferens
– Cranial portion – VD; ED
– Caudal portion -SV; Ejac. Duct
– Communication with rete testis is not
established until 3rd month
Gonadal Descent
• Initial descent in both
sexes relies on the
gubernaculum
– Forms in the peritoneal
folds during 7th week
– Superior end is attached to
gonad, inferiorly it is
attached to fascia between
the external and internal
obliques
• Processus vaginalis
– Evagination of peritoneum
Testicular Descent
Testicular Descent
• Layers encountered by processus
vaginalis
– Transversalis fascia
– Muscle fibers of internal
oblique (cremasteric)
– Thin layer of external oblique
muscle (external spermatic
fascia)
• Descent of testicle
– At level of internal ring by 3rd
month
– Complete descent to scrotum
between 7th-9th month
Normal Testicular Anatomy
Normal Anatomy
Duct System
Blood supply
Blood Testis
Barrier
Spermatic cord supplies
• Blood Supply
• Lymphatics
– Upper lumbar LN
• Nerve supply
– Sympathetic- T10-11
– Unable to differentiate
– Suggested to increase
or decrease dilation
Normal Anatomy
Embryologic Remnants
Normal Histology
Normal Seminiferous Tubule
Cryptorchidism
• Means hidden testis
• Testis develops
intraabdominally,
disorders of descent are
common
• No geographic or ethnic
distributions
• Testes descend to cooler
scrotum to facilitate
spermatogenesis in
humans
Embryology of Testis Descent
• Germ cells migrate from yolk sac to genital ridges at 6
weeks
• Testis differentiation begins to occur at 7 weeks as SRY
protein is produced
• Testosterone and MIF are secreted by testis during week 8
• Testosterone is controlled by maternal hCG, causes
wolffian differentiation into epididymis and vas
• MIF is produced by Sertoli cells and causes mullerian
regression=>appendix testis
Embryology of Testis Descent
(con’t)
• Growth of the gubernaculum is believed to be
controlled by testis paracrine function
• Testis is never more than 1.3 mm from internal
ring at any point in its development
• Testis remain in position but processus
vaginalis extends into scrotum from 12th week
to 7th month
Embryology of Testis Descent
(con’t)
• At 7 months, the vas and testicular vessels increase in
size, the gubernaculum swells, and the processus extends
into scrotum completely
• As the gubernaculum swells, the inguinal canal and
scrotum stretch=>testis rapidly descends
• The epididymis precedes the testis into the scrotum
• Canal portion of processus obliterates, testicular portion
persists as tunica vaginalis
• Gubernaculum atrophies
Incidence of Testicular Descent
• Of 1500 full term infants, 3.4% had true UDT
• Prematurity may predispose to UDT because birth occurs
before descent
• Of 142 premature infants, 30.3% had UDT
• Low birth weight also predisposes to UDT, as weight is
gained, testes descend
• At 1 y.o., 0.8-1.5% have UDT, 75% of full-term and
95% of premature UDT descend during first year
• Most descend during first 3 months after birth probably
due to elevated serum androgens during first 3 months
Incidence of Testicular Descent
(con’t)
• Cremasteric reflex is most active during 2-7 years
old=>retractile testes are misdiagnosed as UDT
– Estimates of UDT at 5 y.o. are about 10%
•
•
•
•
•
Testes do not ascend postnatally
Incidence of UDT in adulthood is < 1%
Absence of one or both testes on exploration is 3-5%
10% of patients have bilateral UDT
14% of patients with UDT have a family member with
UDT
Theories Regarding the
Mechanisms of Testicular Descent
• Traction of the testis by gubernaculum and cremasters
– Severing the gubernaculum does not stop descent in animals
– Weak attachments between gubernaculum and scrotum
• Differential growth of the body wall in relation to
immobile gubernaculum
– Proximity to internal ring is maintained as body grows
– Gubernaculum swells and grows faster than the body
• Intraabdominal pressure pushes the testis through the
inguinal canal
– Increased pressure promotes descent supported by theoretical
and experimental data (Schechter 1960, Bergin 1970, Gier 1970)
Theories Regarding the
Mechanisms of Testicular Descent
(con’t)
• Development and maturation of the epididymis
contributing to descent
– Probably not as important as initially thought
• Effects of the genitofemoral nerve
– Severing the nerve prevents descent in rodents
Endocrine Aspects of UDT
• Exact mechanism of androgen induction of descent is
unknown
• No consistently discernable abnormalities have been
identified in patients w/ UDT
– Conflicting data on baseline LH, FSH, testosterone levels and
response to GnRH stimulation
• Endocrine factors probably play a major role in descent
– Testosterone induces testis descent in humans
– Androgens affect the nuclei of the genitofemoral nerve to
release modulating factors for gubernacular development
– Boys with spinal cord defects at or below nuclei of the nerve
have a higher incidence of UDT
– DHT binds to rat gubernaculum
H-P-T Axis Disorders and UDT
Organ
Hormone
Hypothalamus
LHRH
Kallman’s Syndrome
Pituitary Gland
LH (FSH)
Anencephaly
Testosterone
20,22-desmolase
3b-HSD, 17-hydroxylase
17,20-desmolase
17b-HSD
Testis
DHT
Spermatic cord
Gubernaculum
Processus Vaginalis
Steroid-Receptor
Complex
Disorder
Pseudovaginal
perineoscrotal hypospadias
Testicular Feminization
Reifenstein’s Syndrome
Classification of UDT
• Abdominal
– Impalpable by definition
– Usually “peeping”
• Canalicular
– Or “peeping” at external ring
• Ectopic
– 5 major sites corresponding to
gubernacular branches
– Superficial inguinal is most
common
• Rectractile
– Cremaster reflex
– Most common in 5-6 year olds
Whitaker/Kaplan Classification for
UDT
• Palpable
–
–
–
–
Normal
Retractile
Ectopic
Undescended
• Unpalpable
–
–
–
–
Canalicular
Intra-abdominal
Emergent
Absent
• Agenesis
• atrophy
Work Up of UDT
• Must determine if testes present at
birth
• Physical exam is very important to
evaluate retractile testes
– Non-palpable testis is
intraabdominal, intracanalicular,
absent
• Bilateral UDT requires hormonal
evaluation and challenge
– Elevated gonadotropins (FSH)
suggest bilateral anorchia
– Normal serum gonadotropins=>hCG
challenge (2000 IU x 3days)
– No testosterone response indicates
bilateral anorchia
Imaging of UDT
• Herniography-poor sensitivity and specificity
• U/S-good for inguinal testes, not reliable if higher
• CT-may be helpful for bilateral impalpable testes
– Difficult to perform in young children
• MRI-least invasive, most expensive
– Difficult to perform in young children
• Venography-invasive, pampiniform plexus
present=>testis present
– Non-visualized plexus or blindending does not eliminate testis
• Angiography-difficult to perform, high complications
• Overall accuracy of radiologic imaging for UDT = 44%
– PE is 53% accurate by PMD, 84% accuracy by peds GU
Laparoscopy
• Blind-ending vessels indicate
absent testis
• Vessels into the internal
ring=>inguinal exploration,
laparoscopic
orchiopexy/orchiectomy
• Intra-abdominal testis
– Laparoscopic orchiopexy
– 2 stage orchiopexy (FowlerStephens)
– Abdominal exploration
Histology
• UDT contain atrophied
structures with thickened
basement membrane
• Epithelium contains atypical
germ cells
• Smaller seminiferous tubules
with fewer spermatogonia
• Higher level of UDT correlates
with severity of histologic
abnormalities
• Some of the changes can be
seen in the contralateral
normally descended testis
UDT histology
Normal testis histology
Neoplasm and UDT
•
•
•
•
•
•
•
•
10% of testis CA are in UDT
UDT is 35-48x more likely to have malignancy
Incidence of CA in UDT is 1:2550 testis tumors
Abdominal UDT is 4x more likely than inguinal testis to develop
CA
UDT tumors typically occur around puberty
CIS occurs in 1.7% of UTD
Orchiopexy should be performed between 1-1.5 years old
1/5 of testis CA in patients w/ hx of UDT occurs in contralateral
testis
Neoplasm and UDT
• For bilateral UDT, when CA is detected in one testis
there is a 15% chance of developing tumor in the
contralateral testis
– 30% if both were intra-abdominal UDT
• Seminoma is most common CA in UDT
– Embryonal is second most common
• Gonadoblastoma is most common CA in some intersex
disorders which present w/ UDT
– Often coexists with germ cell tumors
– Develop due to deletion on long arm of Y chromosome
Torsion and UDT
• Increased risk for torsion in
UDT due to anatomic
abnormality between testis
and mesentery
• Incidence is greatest after
puberty with increased testis
size
• Tumor that increases size also
increases risk of torsion
• 64% of adult torsion in UDT
patients had germ cell tumor
• Be aware of abdominal pain
and empty hemiscrotum=>
torsed intra-abdominal UDT
Hernia and UDT
• Processus vaginalis
should obliterate
between the 8th
month of gestation
and 1st month of life
• UDT results in
patent processus
vaginalis
• Hernias are found in
90% of patients w/
UDT
Infertility and UDT
• Spermatogenesis is retarded by maldescent
• Bilateral UDT => poor fertility
• Higher UDT => more damage to seminiferous
tubules
• Earlier orchiopexy may improve chances for
recovery of spermatogenesis
• Sperm counts in unilateral UDT are much lower
than normal
– Contralateral testis may also be defective
Associated Anomalies with UDT
• Abnormalities with vas and
epididymis are associated
with UDT
– Extended length of epididymis,
partial atresia, total atresia,
dissociation from testis
• Cystic Fibrosis is associated
with vasal agenesis, UDT is
common
• DES exposure during
pregnancy results in
epdidymal defects and UDT
• Klinefelter’s syndrome
– Sterility the rule although
mosaicism may result in
fertility
• Noonan’s syndrome
– Male Turner’s syndrome
– Occasional fertility but not
passed to offspring
• Prader-Willi Syndrome
– Hypothalamic defect with
obesity, MR, hypotonia,
short stature
Hormonal Treatment
• hCG is given to stimulate Leydig cells to produce
testosterone=>descent of testes
– Success rate is 14-50% with doses ranging from 3K to 40K
IU for daily or weekly injections
– A dose of at least 10K is needed to have an effect, doses >
15K have significant side effects
– No change in testis histology or bone growth
• GnRH is given if basal LH is low and abnormality in
GnRH secretion is suspected
– Perinasal spray of 1.2mg/day is effective to stimulate LH
– 6%-70% success rate
Surgical Treatment
• Primary goal of pexation is to facilitate
examination
• Basic principles of orchipexy are: localization,
mobilization, cord dissection, isolation of
processus, tension-free relocation to scrotum
• Pexation does not reduce risk of cancer
• Orchiopexy should be performed before 2 y.o.
• Orchiectomy is an option for post-pubertal
males and dysgenetic testes
Orchiopexy
• 80% UDT are palpable
• Of non-palpable testes, 45% are absent, 30% are
abdominal, 25% are lower testes missed on
examination
• Orchiopexy is 92% successful for testes below external
ring and 87% for inguinal, 82% for peeping, 74% for
abdominal
• Success rates for different techniques are 89% for
inguinal exploration, 84% for microvascular repair,
81% for transabdominal, 73% for two-stage, 77% for
staged F-S repair, 67% for standard F-S repair
Standard Orchiopexy
• Transverse inguinal incision, watch for
testis
• Identify testis and divide gubernaculum
• Open tunics and evaluate testis
• Open external oblique fascia, avoid nerve
• Mobilize spermatic cord
• Finger dissection to enlarge scrotal cavity
medially
• Incise scrotal skin, create dartos pouch
• Pass clamp through pouch into inguinal
area and bring testis into pouch by
gubernaculum or tunica
• Pex testis with 4-0 vicryl sutures
• Complications: atrophy, retraction,
torsion, hematoma, nerve or vas injury
Fowler-Stephens Orchiopexy
• Testis has three sources of arterial blood flow
• Testicular artery and veins often limit mobilization
• < 1/3 of boys with intra-abdominal testes have long vasal loop
needed for vasal pedicle orchiopexy
• Testes located > 1cm above internal ring do poorly with this
technique
• Approach requires long inguinal incision and vasal loop must be
protected during inguinal dissection
• Must open hairpin turn of vas for length without disrupting blood
supply after dividing spermatic vessels
• Clamp testicular artery before ligation to ensure collateral blood
supply, incising tunica may be misleading
• Mobilization with wide peritoneal-vasal cuff, may need more direct
exit site
• Same scrotal fixation techniques
Two-Stage Fowler-Stephens
• 84% success rate with staged repair probably due to
improved collateral circulation
• Spermatic vessels are clipped > 1cm cranial to testis,
large clip facilitates identification
• Wait at least 6 months then perform orchiopexy open or
laparoscopically or combined
• Open: patient may not have external ring on affected
side, divide muscles in general area
• Divide vessels above clip, create wide peritoneal cuff
around testis and vas, straighten vasal loop
• Place in dartos pouch through medial incision in
abdominal wall
Laparoscopy
• 95% sensitive for identifying
and/or locating NPT
• Three likely findings:
– blind-ending vessels
– cord structures entering the ring
– intra-abdominal testis
• One-stage lap orchiopexy
brings testis medial to inferior
epigastric vessels into pouch
(Prentiss)
• If vessels and vas enter closed
internal ring, controversy about
groin exploration
• If internal ring is open, can
complete orchiopexy
laparoscopically or open
Laparoscopic view of intra-abdominal
testis at internal ring
Vanishing Testis
• Laparoscopic view of closed internal inguinal ring. A, vas deferens
and atretic spermatic vessels in vanishing testis syndrome. B,
ipsilateral normal vas deferens and spermatic vessels in contralateral
vanishing testis syndrome.
Algorithm for UDT