Spasticity Update
advertisement

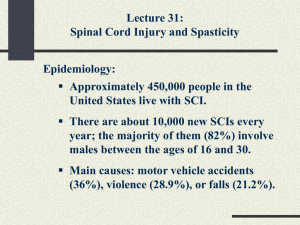
Spasticity Update Michael Saulino, MD PhD Physiatrist MossRehab Assistant Professor Physical Medicine and Rehabilitation Thomas Jefferson University Hospital CME Disclosures 1 • Speaker’s bureau for Jazz Pharmaceuticals • Speaker’s bureau and clinical investigator for Medtronic, Inc • Speaker’s bureau for Ipsen • Consultant for SPR therapeutics and Myoscience CME Disclosures 2 • Will discuss off label and investigational indications for medications and devices • All activities are reviewed by Albert Einstein Healthcare Network’s conflict of interest committee • Honoraria are paid directly to PMR department Synergistic Model of Spasticity Management Removal of Noxious Stimuli Physical Measures Surgery ITB Oral Medications Neurolytic Blockade Synergistic Model of Spasticity Management Removal of Noxious Stimuli Physical Measures Surgery ITB Oral Medications Neurolytic Blockade Oral Pharmacological Options • • • • • • Baclofen (Lioresal) * Diazepam (Valium) Neurontin (Gabapentin) Clonidine (Catapress) Tizadine (Zanaflex) * Dantrolene (Dantrium) * * FDA approved for spasticity Baclofen (Lioresal) • Racemic GABA B receptor agonist • Both pre- and post-synaptic activity resulting in inhibition of both monosynaptic and polysynaptic reflexes at the spinal level • R enatomier considered to the 5-6 times more potent Baclofen Several pharmacologic limitations: Only absorbed in the upper small intestine Saturable active transport mechanism Short half life (3-4 hrs) Requires frequent administrations for effectiveness Sustained release formulation unachievable Arbaclofen Placarbil • Improved absorption of R enatomoer by disguising it as a nutrient which uses an active transport mechanism • Compared with immediate-release baclofen, arbaclofen has a flatter pharmacokinetic profile and more sustained plasma levels allowing less frequent dosing Arbaclofen SCI Trial • Double-blind, placebo-controlled crossover study • 37 SCI patients >1 year after injury, Ashworth > 2 after washout • Arbaclofen 10, 20, or 30 mg BID or placebo • Primary end point: Difference in Ashworth scale Arbaclofen SCI Trial Arbaclofen SCI Trial Arbaclofen SCI Trial Arbaclofen Initial SCI trial suggests efficacy of BID dosing and improved tolerability compared to immediate release racemic oral baclofen Ongoing trial in multiple sclerosis • Currently ongoing at 35 sites in US • Randomizing 200 patients • 4 treatments: placebo, 15 mg, 30 mg, or 40 mg twice daily over 8 weeks • Followed by an on open label study Botulinum Neurotoxin • The most potent neurotoxin known • Derived from Clostridium botulinum • Variations in the polypeptide sequence produce different serotypes of toxin, referred to as types A, B, C1, D, E, F and G • The different serotypes can evoke different immune responses; for each serotype, multiple packaging and formulation options exist, adding to the variability Mechanism of action Mechanism of action Toxin Formulations Available in US • Only Onabotulinumtoxin A (Botox) is approved for adult upper extremity spasticity Toxin Dilution Reasonable Max Dosing 25-100 units/ml 400 U / limb 600 U / session Abobotulinumtoxin A 200-500 units/ml 1500 U / limb 2000 U / session Incobotulinumtoxin A 25-100 units/ml 400 U/ limb 500 U / session Rimabotulinumtoxin B 5000 units/ml 10,000 U / limb 20.000 U / session Onabotulinumtoxin A Intrathecal baclofen therapy • Traditionally, effects of ITB have been related to two factors: – Catheter tip location – Dosage administered • Emerging data suggests that drug concentration / volume administered / flow rate can play a role in therapeutic effects. Visual example of volume effects 1 mL test dose 0.1 mL 0.048 mL Concentration effects • Bernards and colleagues have demonstrated enhanced distribution of ITB away from the catheter tip at lower concentrations in an animal models • Same group also demonstrated logarimithic relationship between ITB concentration and baricity Agent • Chiodo and colleaues reported improved spasticity control in a small case series with lower ITB concentration • Stokic and Yablon demonstrated enhanced electrophysiological suppression of spasticity at lower ITB concentrations • van des Plas and colleagues failed to detect a difference in CRPS-dystonia reduction with differential flow rate and fixed ITB dosing Agents • Intrathecal Lioresal – FDA approved – Medtronic / Novartis • Intrathecal Gablofen – FDA approved – CNS therapeutics • Compounded baclofen – not FDA approved – state regulated, compounding pharmacies Agent • 2 published articles (Moberg-Wolff and Farid et al) describe considerable variability in compounded baclofen compared to branded intrathecal Lioresal • Handful of conference abstracts describing adverse effects with compounded baclofen products