Pap and Gyn Oncology
advertisement

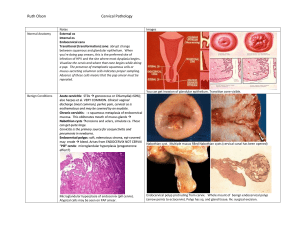
Gyn Oncology Tory Davis, PA-C Introduction Facts: – Mortality from cervical cancer has been reduced by 70% since the advent 50 years ago of the pap smear. – Between 1980-1990, Maine’s cervical cancer mortality rate was ranked third in the nation. Now we’re fourteenth in the US among Caucasians. – Risk of developing cervical cancer over a lifetime is 3.7% among never screened vs 0.3% of those screened regularly Frequency of Pap test screening: ACS and ACOG’s recommendation: – Annual pap and pelvic exam Start at age 21 Under the age of 30 – Pap smears q 2 years – Follow up ASC-US with reflex HPV test Age 30-64 and over – Pap smears plus HPV DNA test every three years if results are negative. If results are discordant, additional testing will be done Stop at age 65-70 if 3 nl paps in a row and no cancer in >10 years No more pap after hysterectomy for benign indication, unless hx of cervical cancer Risk factors for cervical malignancies – Women whose male sexual partners have had other sexual partners with cervical cancer – Women with current or prior HPV infection – Women with current or prior HSV infection – Women who are infected with HIV or other STIs – Women who were DES-exposed in utero – Women with immunosuppression – Smokers and abusers of other substances, including alcohol – Women who have a history of gyn cancer – Women of lower socioeconomic status Human Papilloma Virus Most common STI world-wide Estimated that 6.2M people in the US become infected with the virus annually Lifetime chance of HPV infection is proposed to be 80%-85% in sexually active individuals. > 100 types of HPV, most are harmless. Some (HPV 6 and HPV 11) cause genital warts, but not cancer. High risk HPV for cancer include: 16 &18, which cause >70% of all cervical cancers – Also 31,33,35,39,45,51,52,56,58,59,68,and 69 Human Papilloma Virus New(ish) vaccine (Gardasil) for HPV 6, 11, 16, 18 NB- Even people who contract a highrisk HPV will not necessarily develop cancer. Normal Cervix Clear comprehension of the normal cervix aids understanding abnormal alterations of the cervix. Each cervix demonstrates a unique stage of dynamic epithelial transformation that occurs throughout a women’s life. The physiologic landscape of the normal cervix enables malignant transformation to occur when the conditions are appropriate. Anatomy of the Cervix Cervix: – distal portion of the uterus – length - 3.5 cm; diameter 2.5 cm – endocervical canal - extends from the internal to external os – ectocervix - outside of the cervix, seen during speculum exam Normal Cervix Epithelial cells: – Columnar - mucous secreting epithelium covering the proximal and midendocervical canal – Original squamous epithelial layercovering the vagina and distal ectocervix – Squamocolumnar Junction - boundary between the squamous and columnar epithelium Normal Cervix (continued) Squamocolumnar Junction – may occur anywhere on the ectocervix or endocervix – Starts in endocervix and “rolls out” to ectocervix around puberty – may change at various times as a result of hormonal variations sexual activity pregnancy Normal Cervix (continued) Eversion - seen as a red area on the outside of the cervix as a result of the columnar epithelium. As the uterus and cervix grow during puberty and adolescence, the original SCJ “rolls out”, or everts from inside to outside the cervix. Also seen during pregnancy and with oral contraceptives. Transformation Zone - area between the original and new SCJ. – In the menopausal years, the uterus and cervix decrease in size, and the new SCJ comes to lie upward into the endocervical canal, often out of direct visual contact. Obtaining the Optimal Pap Obtain a pap smear: – Mid cycle – No intercourse or douching x 48-72 hours – No intravaginal medications within the past 72 hours Technique of Cytological Screening Important steps in obtaining an adequate sample: – Collect cells prior to bimanual exam – Avoid contaminating the sample with lubricant (controversial) – Pap first, STD testing after – View entire cervix before sampling – Carefully remove large amounts of discharge before sampling, so you can see the cervix Technique of Cytological Screening Important steps in obtaining an adequate sample: – Do not sample if heavy menses – Treat vaginitis first (if pt likely to come back for the pap) to avoid inflammation-related ASCUS – Obtain the ectocx first and then the endocx to avoid bleeding from the brush – Develop system to minimize fixation time or use liquid-based (ie: Thin Prep) – Gently rotate brush for endocervical sample False Positives False Positive – Vigorous use of the endocervical brush – Airdrying artifact if not using liquid medium – Severe atrophy – Previous irradiation or chemotherapy – Infection/inflammation/repair False Negatives Smears lacking diagnostic cells Sampling errors – inadequate technique – failure to obtain an adequate number of cells – failure to sample TZ component (no endocx cells) Inappropriate technique in spreading cells on slide or incomplete mixing Non-shedding lesion Screening or interpretive error Pap Test Screening Techniques Glass slide, traditional Liquid - based system – Thin prep Advantages: less mucous, fewer thick cell clumps higher rate of dx abnormalities and lower rate of unsatisfactory Disadvantages: laboratory processing is more labor intensive with increase of $15-$20 more than conventional methods Pap results Reported by The Bethesda System But first, some history… – Dysplasia – CIN categories CIN Previously called “dysplasia” Cervical Intraepithelial Neoplasia Infection with HPV Disordered growth of epithelial lining of cervix Mild= CIN I Moderate= CIN II Severe= CIN III Dysplasia “Disordered growth” Disruption of the ordered growth of cervical cells Normal distribution: – bottom layer is made of round young cells. – as cells mature they rise to the surface and flatten out, so that on the surface the cells are flat. Normal Moderate dysplasia In dysplasia and carcinoma-in-situ all of the abnormalities are confined to the surface lining (or "skin") of the cervix – In invasive cancer, cells invade tissue under the surface. NB the difference. Bethesda ASCUS – ASC-H LSIL HSIL AGUS Why doesn’t the Bethesda system include an “invasive cancer” option? ASCUS Atypical cells of unknown significance (abnl cells not quite dysplastic, CIN I) Usually caused by mild infection or inflammation 1 in 20 paps 80% are of ASCUS paps are normal – 13% will be low grade, 7% high grade lesions Next step: test for oncogenic HPV strains LSIL or LGSIL Low grade intraepithelial lesion Encompasses HPV/mild dysplasia/CIN 1 1/600 may become cancer 50% resolve spontaneously Management age-dependant – Under age 20, repeat cytology 12 months – Otherwise, colposcopy HSIL/ HGSIL High-grade intraepithelial lesion Encompasses moderate and severe dysplasia, CIN 2 and CIN 3, CIS 1% risk of cancer Colposcopy with biopsy – Followed by destruction of abnormal tissue (if confirmed and not resolved by biopsy) AGCUS Atypical glandular cells of undetermined significance – “glandular cells that show nuclear atypia appearing to exceed reactive or reparative changes but lacking unequivocal features of adenocarcinoma.” (Bethesda System) – May include endocervical cells, endometrial cells 0.2-0.8% of all paps 20-50% have more serious lesion Must have colposcopy, possibly cervical cone biopsy Cervical Changes Carcinogen Exposure - cause an abnormal maturation process at the transformation zone and begins the process of intrepithelial neoplasia. – Approximately 95% of squamous intrepithelial neoplasia occurs within the transformation zone. Cigarette smoking Intercourse at a young age HPV (16,18,31,33,35,39,45,51,52,56,and 58) Management of Abnl Pap See algorithm pdf Invasive Cervical Cancer US: 10k cases/year with <4k deaths/year Worldwide: 370,000 cases/year with 50% mortality rate Prognosis dependent on stage at diagnosis Cervical Cancer and CIN Treatments Local Excision- ie: biopsy, usually done during colposcopy Cryocautery- destroy abnl tissue by freezing Laser Therapy LEEP- Loop Electrosurgical Excision Procedure Cone biopsy Hysterectomy VIN Vulvar Intraepithelial Neoplasia 80% positive for HPV (usually HPV-16) Assoc with smoking MC CC: pruritis Multicentric origin (as opposed to single point origin of cervical cancer) VIN Lesions vary in appearance- single, multiple, flat, raised, papules, white, red…. 1-2% of women with cervical dysplasia have multifocal ds involving vagina, vulva, perineum, perianal Range- mild dysplasia to carcinoma in situ Dx with colposcope and bx Vulvar Cancer 5% of gynecological cancers Postmenopausal women Long hx vulvar irritation, pruritis, bloody discharge Lesions: early look like dermatitis, late can be indurated, cauliflower-like or ulcerated Dx with bx Vaginal Cancer 3% of gyn cancers Early- asymptomatic Then painless bleeding from ulcerated tumor Late vaginal bleeding, pain, wt loss 85% squamous cell cancers Endometrial cancer US lifetime risk 1.3% (Black women) 2.4% (Caucasian) Usually diagnosed age 60s, but can occur in 20-30 year olds 25% pre-menopausal Abnormal endometrial bleeding Estrogens and Endometrial Ca Hyperestrogenism is a causative factor – Estrogen unopposed by progesterone, ie: PCOS, chronic anovulation, delayed menopause – Exogenous estrogens – Nulliparity Estrogens stimulate endometrium, progesterones are anti-proliferative Endometrial cancer not related to sexual activity Presentation Abnl vaginal (endometrial) bleeding, usu post-menopausal No screening program Physical exam unremarkable Endocervical cytology MAY reveal carcinoma Better results with aspiration cytology or endometrial bx Testing Pap- Presence of endometrial cells on cervical smear of post-menopausal women assoc with endometrial cancer in 2-6 % asx women and up to 25% of women with post-menopausal bleeding Hysteroscopy- can increase dx accuracy. Also can promote transtubal spread to peritoneal cavity CT- pelvic anatomy MRI- identifying myometrial invasion CA-125 Well-established tumor marker for ovarian cancer May be elevated in endometrial cancer as well NOT for screening Limited use for management Endometrial ca tx Surgical- hysterectomy, bilateral salpingo-oophorectomy, staging (pelvic and peri-aortic lymphadenectomy) Radiation can be curative, but 20% lower cure rate than surgical, (so if it were MY mom, I’d go with surgery) Ovarian Cancer 3-4% of cancer in women 4th most-frequent cause of cancer deaths in women Why? Lifetime risk of developing ovarian cancer 1.4% Lifetime risk of death from ovarian cancer 1% Etiology 90% sporadic, 10% genetic predisposition Incessant ovulation lots of opportunity for gene mutations Protective: OCPs, pregnancy, hx breastfeeding…Why? Also bilateral tubal ligation…why? Risks: diets high in fats, increased BMI Presentation Insidious Non-specific GI complaints – Nausea, dyspepsia, altered bowel habits, early satiety, sensation of pelvic weight Menstrual abnormalities- in 15% of reproductive-aged patients Dx Suspicion, good history, good exam – 10% of masses <10 cm missed on physical exam – Attention to nodes: supraclavicular, inguinal and umbilical- Sister Mary Joseph node Pelvic US: good sensitivity, poor specificity CA 125- not as screening for low-risk women, but good for dx – Especially coupled with ultrasound – Low CA-125 does NOT exclude the dx CT can delineate retroperitoneal structures MRI best for info about the nature of the neoplasm Ovarian Cancer Tx Surgical Consult gynecological oncologist, who can address both medical and surgical needs Questions?