New Frontiers and Treatment Paradigms for
Stroke Prevention in
Atrial Fibrillation
Evidence- and Guideline-Based Strategies for Optimizing
Clinical Outcomes and Anticoagulation-Based
Management for SPAF
Program Chairman
Samuel Z. Goldhaber, MD
Cardiovascular Division
Brigham and Women’s Hospital
Professor of Medicine
Harvard Medical School
Welcome and Program Overview
CME-certified symposium jointly
sponsored by the University of
Massachusetts Medical School and
CMEducation Resources, LLC
Commercial Support: This National
Initiative is Sponsored by an
Independent Educational Grant
from the Bristol-Myers
Squibb/Pfizer Cardiovascular
Partnership.
Program Faculty
PROGRAM CHAIRMAN
SAMUEL Z. GOLDHABER, MD
Cardiovascular Division
Brigham and Women’s Hospital
Professor of Medicine
Harvard Medical School
CHRISTIAN T. RUFF, MD, MPH
TIMI Study Group
Brigham and Women’s Hospital
Harvard Medical School
Boston, MA
ELAINE M. HYLEK, MD, MPH
Professor of Medicine
Department of Medicine
Boston University Medical Center
Boston, Massachusetts
Conflict of Interest Disclosures
Program Chairman
SAMUEL Z. GOLDHABER, MD
Research Support: Eisai, EKOS, Johnson & Johnson, sanofi-aventis
Consultant: Baxter, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi,
Eisai, Janssen, Merck, Pfizer, Portola, sanofi-aventis
CHRISTIAN T. RUFF, MD, MPH
Research Support: Daiichi Sankyo, AstraZeneca, Bristol-Meyers Squibb,
sanofi-aventis
Consultant: Alere and Beckman Coulter
ELAINE M. HYLEK, MD, MPH
Research Support: Bristol-Myers Squibb, Ortho-McNeil
Consultant: Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi
Sankyo, Johnson & Johnson, Pfizer
New Paradigms in the Science and Medicine of
Stroke Prevention for Atrial Fibrillation
Epidemiology and Overview
Risk, Disease Burden, and Deciphering the Maze
of Risk-Specific Interventions for AF
Focus on Non-Monitored Oral Anticoagulation and the Unmet
Need for Safer and More Effective Stroke Prevention in NVAF
Samuel Z. Goldhaber, MD
Program Chairman
Director, VTE Research Group
Cardiovascular Division
Brigham and Women’s Hospital
Professor of Medicine
Harvard Medical School
Faculty COI Disclosures
Research Support
Eisai, EKOS, Johnson & Johnson, sanofiaventis
Consultant
Baxter, Boehringer-Ingelheim, Bristol-Myers
Squibb, Daiichi, Eisai, Janssen, Merck,
Pfizer, Portola, sanofi-aventis
Formal Definition: Atrial Fibrillation
AF is an arrhythmia characterized by
uncoordinated atrial activation, with
consequent deterioration of atrial
mechanical function
Circulation 2011; 121: e269-e367
The ECG of Atrial Fibrillation
Normal
sinus
rhythm
Atrial
fibrillation
The “3 Ps” and Natural History
of Atrial Fibrillation
Paroxysmal
Persistent
Permanent
Self-Terminating
Lasts > 7 Days
Cardioversion
Failed or Not
Attempted
Normal Sinus Rhythm
Atrial Fibrillation
Paroxysmal AF is as likely
to cause stroke as
persistent or permanent AF
Atrial Fibrillation: Epidemiology
► The No. 1 preventable cause of stroke
► In the United States, up to 16 million
individuals will be affected by the year 2050
► Increasing survival from heart attack and
increasing age (“the ‘graying’ of America”)
help explain rise in incidence of AF
Atrial Fibrillation Risk Factors
Magnani JW et al. Circulation 2011; 124: 1982-1993
Projected Number of People with AF
(millions)
Atrial Fibrillation: An Epidemic
18
16
14
US
Prevalence
16 million
12
1 in 4 lifetime
risk in men and women ≥ 40 years old
10
8
6
4
2
0
Miyakasa Y, et al. Circulation. 2006; 114:119-125.
Year
Prevalence, percent
Relationship Between
Atrial Fibrillation and Age
Age, years
Go AS, et al. JAMA. 2001; 285:2370-2375.
Atrial Fibrillation Causes Stroke
Left Atrial Appendage Thrombus
Chimowitz. Stroke 1993; 24: 1015
Zabalgoitia. J Am Coll Cardiol 1998; 31: 1622
Stroke and Atrial Fibrillation Burden
Approximately 5-fold increased risk of stroke
Quantify stroke risk: CHADS2/ CHA2DS2-VASc
AF strokes have worse outcomes
Costly health care ~ $16 billion/year
30
Framingham
20
AF prevalence
10
Strokes attributable
to AF
%
0
50–59
60–69
70–79
Age Range (years)
Wolf PA, et al. Stroke 1991; 22: 983-988
80–89
Ischemic Strokes in Atrial Fibrillation
More Likely to be Severely Disabling
Framingham Heart Study
73
58
36
33
16
Lin HJ, et al. Stroke. 1996;27:1760-1764.
30
16
11
AF PIE:
FUTURE
AF PIE:
PAST
Fuster V. Circulation 2012; epubl April 18
ESC 2012 AF Update Guidelines
Assess stroke risk exclusively with CHA2DS2VASc and no longer use CHADS2
ESC Guidelines recommend anticoagulation
for stroke prevention with CHA2DS2-VASc
score of 1 or greater
Preference given to novel, non-monitored
anticoagulants: apixaban, rivaroxaban, and
dabigatran
Anticoagulation in Atrial Fibrillation
Effects on Stroke Risk Reduction
Warfarin better
Control better
AFASAK
RRR of stroke:
62%
SPAF
BAATAF
CAFA
SPINAF
RRR All-cause mortality:
26%
EAFT
Aggregate
100%
50%
RRR, relative risk reduction.
Hart RG, et al. Ann Intern Med. 1999;131:492-501.
0
-50%
-100%
ESC 2012 Update Guidelines
HAS-BLED for Evaluation of Bleeding Risk
Clinical Characteristic
Hypertension (systolic BP > 160 mm Hg)
Abnormal renal or liver function
Points
1
1+1
Stroke
1
Bleeding
1
Labile INRs
1
Elderly (age > 65 years)
1
Drugs or alcohol
1+1
Maximum score
9
Pisters R, et al. Chest. 2010;138:1093-1100.
Swedish AF Cohort; Circulation 2011; 125: 2298-2307
Known Problems With Warfarin
1) Delayed onset/offset
2) Unpredictable dose response
3) Narrow therapeutic index
4) Drug-drug, drug-food interactions
5) Problematic monitoring
6) High bleeding rate
7) Slow reversibility
Warfarin Will Likely Survive: Why?
1)
Established efficacy
2)
Low cost ($4/month; $10/3 mos)
3)
Long track record (1954)
4)
Centralized anticoagulation clinics that maintain
TTRs > 60%
5)
Rapid, turnaround genetic testing
6)
Point-of-care self-testing
7)
INR testing q 12 weeks if stable
CoumaGen-II. Circ 2012; March 19
ACCP Chest Guidelines 2012
COUMAGEN-II
Pharmacogenetic Dosing Achieves TTR of 71%
Circulation 2012; epub March 19
Comparison Overview of New
Anticoagulants with Warfarin
Features
Warfarin
New Agents
Onset
Slow
Rapid
Dosing
Variable
Fixed
Yes
No
Many
Few
Yes
No
Half-life
Long
Short
Antidote
Yes
No
Food effect
Drug interactions
Monitoring
Sites of Action in Coagulation System
Novel Factor Xa and DT Inhibitors
Steps in Coagulation
Pathway
Drugs
TF/VIIa
Initiation
X
IX
VIIIa
IXa
Va
Propagation
II
Rivaroxaban
Apixaban
Edoxaban
Betrixaban
IIa
Dabigatran
Xa
Fibrin formation
Fibrinogen
Hankey GJ and Eikelboom JW. Circulation 2011;123:1436-1450
Fibrin
Novel Oral Anticoagulants
Important Comparative Features
Dabigatran
Rivaroxaban
• Oral direct thrombin inhibitor
• Twice daily dosing
• Renal clearance
• Direct factor Xa inhibitor
• Once daily (maintenance), twice daily (loading)
• Renal clearance
Apixaban
• Direct factor Xa inhibitor
• Twice daily dosing
• Hepatic clearance
Edoxaban
• Direct factor Xa inhibitor
• Once daily dosing
• Hepatic clearance
Circulation 2010;121:1523
Comparison of Phase 3 SPAF Trials
for NOACs: A Robust Trial Base
Novel Anticoagulants
Fxa Inhibitor
FIIa Inhibitor
Dabigatran
Rivaroxaban
Apixaban
Edoxaban
Open Label
Double Blind
Double Blind
Double Blind
Two Doses
Two Doses
Two Doses
Two Doses
Twice Daily
Once Daily
Twice Daily
Once Daily
RE-LY
ROCKET-AF
ARISTOTLE
ENGAGE
“Best Options” for Anticoagulation
The Consensus is Shifting
Despite continued use of warfarin, NOACs are
considered by many professional medical
organizations to be the “best option” for
anticoagulation of SPAF patients:
►
ESC 2012 AF Update Guidelines
►
ACCP 2012 Guidelines
►
Canadian AF Guidelines
ESC 2012
UPDATE
GUIDELINES
For
ATRIAL
FIBRILLATION
The Rationale for AF Registries
Registries provide a “real life” perspective on patient
populations, management “in the field,” and
outcomes in settings that do not have the special
resources and monitoring capabilities of pivotal
randomized clinical trials.
Information from registries complements clinical trial
data.
Registries can highlight the disconnect between
evidence/guidelines and clinical practice.
The GARFIELD Registry
►
Novel approach to outcomes research
►
Planned to be conducted in 50 countries
►
50,000 prospective and 5000 retrospective patients
►
Patients newly diagnosed with non-valvular AF
►
Five sequential cohorts
►
Random site selection
►
Sites representative of national AF care settings
►
Consecutive patients
►
Minimum follow-up period of 2 years
Summary of Garfield Data
Cohort One: ESC 2012
►
10,537 were available for this analysis
• 5075 retrospective and 5462 prospective
►
Newly diagnosed patients carry high risk for stroke
●
●
►
57% with CHADS2 score >2
83% with CHA2DS2-VASc score >2
VKAs not prescribed in:
●
●
38% of patients with CHADS2 score >2
40% of patients with CHA2DS2-VASc score >2
Modest Use of Vitamin K Antagonists
Even in High-Risk Patients
European Heart Survey
OAC therapy (%)
100
5333 AF patients in 35 countries: 2003–2004
80
60
58
59
1
2
64
61
3
4
40
20
0
CHADS2 score
OAC, oral anticoagulant
Nieuwlaat et al. Eur Heart J 2006; Gage et al. JAMA 2001
A “Failure to Prophylax” Syndrome
Over the past decade, about 40% of patients
with atrial fibrillation are unprotected from
stroke because of failure to prescribe
anticoagulation.
Because criteria for anticoagulation have
expanded in 2012, the problem has intensified.
Heightened awareness of the disconnect
between guidelines/evidence and suboptimal
intervention for SPAF. Anticoagulation is
necessary as a first step.
The SPAF Landscape 2012: Conclusions
► The frequency of atrial fibrillation is increasing,
so risk of devastating stroke is increasing as
well.
► Anticoagulants can effectively reduce stroke
risk, but they are underutilized.
► NOACs have less ICH bleeding risk than
warfarin and are superior—or at least
noninferior—for stroke prevention.
► We must overcome the failure-to-prophylax
syndrome.
New Paradigms in the
Science and Medicine of Heart Disease
State-of-the-Art Risk Stratification of
Patients with Atrial Fibrillation
Anticoagulation Strategies Based on Established and
Evolving Atrial Fibrillation Scoring Systems for
Thrombosis and Hemorrhagic Risk
Elaine M. Hylek, MD, MPH
Professor of Medicine
Department of Medicine
Boston University Medical Center
Boston, Massachusetts
Independent Predictors of Stroke in AF
Systematic Review
Significant by
Multivariate Analysis
Adjusted Relative Risk
(95% CI)
Prior stroke or TIA
5 of 5 studies
2.5 (1.8–3.5)
Increasing age
6 of 6 studies
1.5/decade (1.3–1.7)
History of hypertension or
systolic BP > 160 mm Hg
5 of 5 studies
2.0 (1.6–2.5)
Diabetes
4 of 4 studies
1.8 (1.5–22)
Female gender
3 of 6 studies
1.6 (1.4–1.9)
Heart failure
0 of 4 studies*
Not significant
Coronary artery disease
0 of 4 studies
Not significant
* Significant in a subgroup of participants undergoing echocardiography in
trials included AFI pooled analysis
Hart RG et al. Neurology 2007; 69: 546.
Nonvalvular Atrial Fibrillation
Stroke Rates Without Anticoagulation
According to Isolated Risk Factors
Prior
Hypertension
Age
Stroke/TIA > 75 years
Hart RG et al. Neurology 2007; 69: 546.
Female
Diabetes Heart Failure
LVEF
Risk Stratification in Atrial Fibrillation
Established Stroke Risk Factors
High-Risk Factors
Moderate-Risk Factors
► Mitral stenosis
►Age > 75 years
►Hypertension
►Diabetes mellitus
►Heart failure or ↓ LV function
► Prosthetic heart valve
► History of stroke or TIA
Less Validated Risk Factors
►
►
►
►
Age 65–75 years
Coronary artery disease
Female gender
Thyrotoxicosis
Singer DE, et al. Chest 2004;126:429S.
Fang MC, et al. Circulation 2005; 112: 1687.
The CHADS2 Score
Stroke Risk Threshold Favoring Anticoagulation
Approximate
Risk Threshold for
Anticoagulation
Score
(points)
Risk of Stroke
(%/year)
0
1.9
1
2.8
3%/year
2
3
4
5
6
Van Walraven C, et al. Arch Intern Med 2003; 163:936.
Go A, et al. JAMA 2003; 290: 2685.
Gage BF, et al. Circulation 2004; 110: 2287.
4.0
5.9
8.5
12.5
18.2
The CHADS2 Score
Stroke Risk Score for Atrial Fibrillation
Score (points)
Prevalence (%)*
Congestive Heart failure
Hypertension
Age > 75 years
Diabetes mellitus
Stroke or TIA
1
1
1
1
2
32
65
28
18
10
Moderate-High risk
Low risk
>2
0-1
50-60
40-50
VanWalraven C, et al. Arch Intern Med 2003; 163:936.
* Nieuwlaat R, et al. (EuroHeart survey) Eur Heart J 2006 (E-published).
CV Event Rates in Patients with Atrial
Fibrillation Related to CHADS2 Score
REACH Registry
Goto S, et al. Am Heart J 2008; 156: 855.
The CHA2DS2-VASc Score
Stroke Risk Score for Atrial Fibrillation
Weight (points)
Congestive heart failure or LVEF < 35%
Hypertension
Age > 75 years
Diabetes mellitus
Stroke/TIA/systemic embolism
Vascular Disease (MI/PAD/Aortic plaque)
Age 65-74 years
Sex category (female)
1
1
2
1
2
1
1
1
Moderate-High risk
Low risk
>2
0-1
Lip GYH, Halperin JL. Am J Med 2010; 123: 484.
Patient Selection for Anticoagulation
Additional Considerations
► Risk of bleeding
► Newly anticoagulated vs established therapy
► Availability of high-quality anticoagulation
management program
► Patient preferences
Published Bleeding Risk Scores
Patients on Oral Vitamin K Antagonist Anticoagulant
Therapy
Low
Kuijer et al.
Arch Intern Med
1999;159:457.
0
Moderate High
1-3
>3
Beyth et al.
Am J Med
1998;105:91.
0
1-2
≥3
Gage et al.
Am Heart J
2006;151:713.
<1
2-3
≥4
Shireman et al.
Chest
2006;130:1390.
≤
1.07
1.07 - 2.19
>
2.19
Tay, Lane & Lip. Thromb Haemost 2008; 100: 955.
1.6 x age + 1.3 x sex +2.2 x cancer; 1 point for
≥ 60 years old, female or malignancy; 0 if none
≥ 65 years old; GI bleed within 2 weeks; prior
stroke; comorbidities (recent MI, Hct < 30%,
diabetes, Cr > 1.5 mg/dL) ;1 point for each
condition; 0 if absent
HEMORR2HAGES score: liver/renal disease,
EtOH abuse, malignancy, > 75 years old, low
platelet count or function, rebleeding risk,
uncontrolled Htn, anemia, genetic factors
(CYP2C9) risk of fall or stroke; 1 point for each
factor; 2 points for previous bleeding
(0.49 x age > 70) + (0.32 x female) + (0.58 x
remote bleed) + 0.62 x recent bleed) + 0.71 x
EtOH/drug abuse) + (0.27 x diabetes) + (0.86 x
anemia) + (0.32 x antiplatelet drug use); 1 point
for each; 0 if none
Advances in the
Science and Medicine of SPAF
Importance of the HAS-BLED Score
Risk Score for Predicting Bleeding in
Anticoagulated Patients with Atrial Fibrillation
Weight (points)
Hypertension (> 160 mm Hg systolic)
Abnormal renal or hepatic function
Stroke
Bleeding history or anemia
Labile INR (TTR < 60%)
Elderly (age > 75 years)
Drugs (antiplatelet, NSAID) or alcohol
1
1-2
1
1
1
1
1-2
High risk
Moderate risk
Low risk
>4
2-3
0-1
(> 4%/year)
(2-4%/year)
(< 2%.year)
Pisters R, et al. Chest 2010; 138: 1093.
Lip GYH, et al. J Am Coll Cardiol 2010; 57: 173.
Canadian Cardiovascular Society
AF Guidelines 2012 Update
Assess Thromboembolic
Risk (CHADS2)
CHADS2 = 1
CHADS2 = 0
CHADS2 > 2
Increasing stroke risk
No antithrombotic
No additional
risk factors of
stroke
ASA
Either female
sex or
vascular
disease
OAC*
Age > 65 y or
combination
of female sex
and vascular
disease
OAC*
*Aspirin is a reasonable
alternative in some as
indicated by risk/benefit
OAC
Canadian Cardiovascular Society
AF Guidelines 2012 Update
All patients with atrial fibrillation or atrial flutter
(paroxysmal, persistent, or permanent), should be
stratified using a predictive index for stroke (eg, CHADS2)
and for the risk of bleeding (eg, HAS-BLED), and that
most patients should receive either an oral anticoagulant
or aspirin. (Strong recommendation, high quality
evidence)
When oral anticoagulation therapy is indicated, most
patients should receive dabigatran, rivaroxaban, or
apixaban* in preference to warfarin.
(Conditional recommendation. high-quality evidence).
*Once approved by Health Canada.
ESC 2012 AF Update Guidelines
2012 focused update of the ESC Guidelines for the management of atrial fibrillation: Europace. 2012 Aug 24
ESC 2012 Guidelines: Identifying
“Truly Low-Risk” Patients with AF
Thus, this guideline strongly recommends a practice shift toward
greater focus on identification of ‘truly low-risk’ patients with AF
(ie,‘age <65 and lone FL’ who do not need any antithrombotic
therapy), instead of trying to focus on identifying ‘high-risk’
patients.
To achieve this, it is necessary to be more inclusive (rather than
exclusive) of common stroke risk factors as part of any
comprehensive stroke risk assessment. Indeed, patients with AF
who have stroke risk factor(s) > 1 are recommended to receive
effective stroke prevention therapy, which is essentially OAC with
either well-controlled VKA therapy [INR 2-3, with a high
percentage of time in the therapeutic range (TTR), for example,
at least 70%] or one of the NOACs
2012 focused update of the ESC Guidelines for the management of atrial fibrillation: Europace. 2012 Aug 24
CHA2DS2-VASc vs. CHADS2
Is More Information Better?
►
►
►
►
The new scoring systems have been adopted in
Europe but not in the United States, even in the
latest practice guideline updates.
The components of the CHA2DS2-VASc score are
less well validated than those of the CHADS2 score.
The C-statistic used to validate the CHA2DS2-VASc
score is only marginally superior to those of other
schema.
There is no consensus about how to combine
stroke risk and bleeding risk scores into a
composite instrument.
Current AF Stroke Risk Stratification Schemes
Limitations, Challenges, and Opportunities
►
All have modest predictive value for thromboembolism
Patients classified as low risk must truly be at low risk to safely avoid
anticoagulation
Should classify small proportion into the intermediate risk category, for
which optimum therapy is less clear
►
Incorporate risk factors as cumulative
►
Should be comprehensive yet easy to apply
►
Scoring systems are the most popular method
Acronym for easy recall
Should be validated in multiple populations, ideally clinical practice
populations, rather than in the control arms of trial cohorts
Risk schemes must evolve to address the
wider therapeutic margin offered by new oral anticoagulants
Lip GYH, Halperin JL. Am J Med 2010;123:484
Atrial Fibrillation and Thromboembolism
The Next Challenges
►
►
►
►
►
Better risk-stratification that balances stroke and
bleeding and addresses new anticoagulants
Noninvasive methods to better predict events and
guide therapy
Safer treatments for the highest risk patients
Achieving and confirming successful rhythm
control over time
Targeted atrial fibrillation prevention
New Paradigms in the
Science and Medicine of Heart Disease
Deciphering the Maze of Evidence from
Landmark Trials Evaluating Non-Monitored,
Oral Anticoagulants (NOACs) for SPAF
Christian T. Ruff, MD, MPH
TIMI Study Group
Brigham and Women’s Hospital
Harvard Medical School
Boston, MA
Faculty COI Disclosures
Research Support
Daiichi Sankyo, AstraZeneca, Bristol-Meyers
Squibb, sanofi-aventis
Consultant
Alere and Beckman Coulter
Properties of an Ideal Anticoagulant
Properties
Benefit
Oral, once-daily dosing
Ease of administration
Rapid onset of action
No need for overlapping
parenteral anticoagulant
Minimal food or drug interactions
Simplified dosing
Predictable anticoagulant effect
No coagulation monitoring
Extra renal clearance
Safe in patients with renal
disease
Rapid offset in action
Simplifies management in case of
bleeding or intervention
Antidote
For emergencies
Major Advances In
Oral Anticoagulation for SPAF
ROCKET AF
(Rivaroxaban)
2010
6 Trials of Warfarin vs Placebo
1989-1993
RE-LY
(Dabigatran)
2009
ENGAGE AF
(Edoxaban)
2013
ARISTOTLE
(Apixaban)
2011
Comparative Pharmacokinetics/
Pharmacodynamics of Novel Agents
Dabigatran
Apixaban
Rivaroxaban
Edoxaban
IIa
(thrombin)
Xa
Xa
Xa
2
1-3
2-4
1-2
None
15%
32%
NR
Bioavailability
7%
66%
80%
> 45%
Transporters
P-gp
P-gp
P-gp/BCRP
P-gp
Protein binding
35%
87%
>90%
55%
12-14h
8-15h
9-13h
8-10h
Renal elimination
80%
25%
33%
35%
Linear PK
Yes
Yes
No
Yes
Target
Hrs to Cmax
CYP metabolism
Half-life
BCRP = breast cancer resistance protein; CYP = cytochrome P450; NR = not reported;
P-gp = P-glycoprotein
Ruff CR and Giugliano RP. Hot Topics in Cardiology 2010;4:7-14
Ericksson BI, et al. Clin Pharmacokinet 2009;48:1-22
The RE-LY Trial: Dabigatran
RE-LY
Atrial fibrillation
≥1 Risk Factor
Absence of contra-indications
951 centers in 44 countries
PROBE=Prospective Randomized
Open Trial with Blinded
Adjudication of Events
R
open
Warfarin
(INR 2.0-3.0)
N = 6022
Blinded
Dabigatran
Etexilate
110 mg bid
N = 6015
Dabigatran
Etexilate
150 mg bid
N = 6076
10 efficacy outcome = stroke or systemic embolism
10 safety outcome = major bleeding
Non-inferiority margin 1.46
RE-LY Efficacy (Dabigatran)
Stroke/Systemic Embolic Event
Non-inferiority Superiority
P-value
P-value
Dabigatran 110 vs Warfarin
< 0.001
Dabigatran 150 vs Warfarin
< 0.001
0.34
< 0.001
Margin = 1.46
Connolly, et al. N Engl J
Med 2009;361:1139-51
0.50
0.75
HR
1.00
1.25
(95% CI)
1.50
RE-LY Efficacy (Dabigatran)
Dabigatran 110 mg
Dabigatran 150 mg
Stroke/SEE
0.91 (0.74-1.11)
0.66 (0.53-0.82)
Ischemic Stroke
1.11 (0.89-1.40)
0.76 (0.60-0.98)
Hemorrhagic Stroke
0.31 (0.17-0.56)
0.26 (0.14-0.49)
Connolly, et al. N Engl J Med 2009;361:1139-51
0.1
0.3
0.5
Dabigatran Better
1.0
2.0
Warfarin Better
RE-LY Safety Results (Dabigatran)
Dabigatran 110 mg
Dabigatran 150 mg
Major Bleed
ICH
GI Bleed
MI
0.80 (0.69-0.93)
0.93 (0.81-1.07)
0.31 (0.20-0.47)
0.40 (0.27-0.60)
1.10 (0.86-1.41)
1.50 (1.19-1.89)
1.29 (0.96-1.75)
1.27 (0.94-1.71)
Connolly, et al. N Engl J Med 2009;361:1139-51
0.1
0.3
0.5
Dabigatran Better
1.0
2.0
Warfarin Better
RE-LY Efficacy Stratified by CHADS2
Annualized Rate Stroke/SEE (%)
CHADS2
D110
D150
WARF
0-1
1.06
0.65
1.05
2
1.43
0.84
1.38
3-6
2.12
1.88
2.68
D150
mg
D110
mg
P=
0.44
0.50
1.00
1.50
Dabigatran
Warfarin
better
better
Oldgren J, et al. ACC 2010
P=
0.50
1.00
Dabigatran
better
0.82
1.50
Warfarin
better
RE-LY Efficacy Stratified by
Prior Vitamin K Anatagonist
Ezekowitz MD, et al. Circulation 2010;122:2246-2253
RE-LY Cardioversion (Dabigatran)
Stroke/SEE
1.8
Major Bleeding
1.7
1.6
1.4
1.2
1
0.8
0.8
0.6
0.6
0.4
0.6
0.6
0.3
0.2
0
Dabi 110 mg
Dabi 150 mg
(N = 647)
(N = 672)
Nagarakanti R, et al. Circulation 2011;123:131-136
Warfarin
(N = 664)
Dabigatran Approval
Prevention of stroke in AF
Available in 75 mg and 150 mg
(twice daily)
Dose of 75 mg if CrCl 15-30 mL/min
Data in favor of 110 mg were
“suggestive, but not entirely
convincing”
ROCKET AF: Rivaroxaban
Risk Factors
• CHF
• Hypertension
At least 2
• Age 75
required
• Diabetes
OR
• Stroke, TIA or Systemic
embolus
Atrial Fibrillation
Rivaroxaban
20 mg daily
15 mg for Cr Cl 30-49
Randomize
Double blind / Double Dummy
(n = 14,266)
Warfarin
INR target - 2.5
(2.0-3.0 inclusive)
Monthly Monitoring and adherence to
standard of care guidelines
Primary End point: Stroke or non-CNS Systemic Embolism
Statistics: non-inferiority, > 95% power, 2.3% warfarin event rate
ROCKET AF Efficacy
Stroke/Systemic Embolic Event
Rivaroxaban Warfarin
On
Treatment
Event
Rate
Event
Rate
HR
(95% CI)
P-value
1.70
2.15
0.79
(0.65, 0.95)
0.015
2.12
2.42
0.88
(0.74, 1.03)
0.117
N = 14,143
ITT
N = 14,171
0.5
1
Rivaroxaban
better
2
Warfarin
better
Event Rates are per 100 patient-years
Based on Safety on Treatment or Intention-to-Treat through
Site Notification populations
Patel, et al. N Engl J Med 2011;365(10);883-891
ROCKET AF Key Secondary Efficacy
Rivaroxaban
(%/yr)
Warfarin
(%/yr)
Hazard Ratio
(95% CI)
Pvalue
Ischemic Stroke
1.34
1.42
0.94 (0.75-1.17)
0.581
Hemorrhagic Stroke
0.26
0.44
0.59 (0.37-0.93)
0.024
MI
0.91
1.12
0.81 (0.63-1.06)
0.121
Total Mortality
1.87
2.21
0.85 (0.70-1.02)
0.073
Vascular Mortality
1.53
1.71
0.89 (0.73-1.10)
0.289
Event
Patel, et al. N Engl J Med 2011; 365(10);883-891
ROCKET AF Safety (Rivaroxaban)
Rivaroxaban
(%/yr)
Warfarin
(%/yr)
Hazard Ratio
(95% CI)
Pvalue
Major and Clinically
Relevant Bleed
14.9
14.5
1.03 (0.96-1.11)
0.44
Major Bleed
3.6
3.4
1.04 (0.90-1.20)
0.58
Fatal Bleed
0.2
0.5
0.50 (0.31-0.79)
0.003
ICH
0.5
0.7
0.67 (0.47-0.93)
0.02
Event
Patel, et al. N Engl J Med 2011; 365(10);883-891
ROCKET AF Efficacy (Rivaroxaban)
Moderate Renal Impairment
Fox KA, et al. Eur Heart J 2011;32(19):2387-94.
ROCKET AF Safety
Moderate Renal Impairment
Fox KA, et al. Eur Heart J 2011;32(19):2387-94.
Rivaroxaban Approval
Prevention of stroke in AF
Dose 20 mg if CrCl > 50 mL/min
Dose of 15 mg if CrCl 15-50 mL/min
AVERROES
AVERROES Trial Design: Apixaban
36 countries, 522 centers
AF and >1 risk factor, and
demonstrated or unexpected
unsuitable of VKA
R
Apixaban 5 mg bid
2 mg bid in selected patients
5600 patients
Double-blind
ASA (81-324 mg/d)
Primary Outcome: Stroke or
Systemic Embolic Event (SEE)
AVERROES: Apixaban
Stroke or Systemic
Embolic Event
Major
Bleeding
0.020
0.05
0.04
Aspirin
0.03
P < 0.001
Apixaban
0.01
0.00
3
6
P < 0.001
0.010
0.02
0
Apixaban
0.015
9
12
18
HR 0.45 (0.32-0.62)
Connolly SJ, et al. N Engl J Med 2011 (epub)
Aspirin
0.005
0.000
0
3
6
9
12
HR 1.13 (0.74-1.75)
18
ARISTOTLE: Apixaban
ARISTOTLE Trial Design: Apixaban
Inclusion risk factors
Age ≥ 75 years
Prior stroke, TIA, or SE
HF or LVEF ≤ 40%
Diabetes mellitus
Hypertension
Randomize
double blind,
double dummy
(n = 18,201)
Exclusion
Mechanical prosthetic valve
Severe renal insufficiency
Need for aspirin plus
thienopyridine
Apixaban 5 mg oral twice daily
Warfarin
(2.5 mg bid in selected patients)
(target INR 2-3)
Warfarin/warfarin placebo adjusted by INR/sham INR
based on encrypted point-of-care testing device
Primary outcome: stroke or systemic embolism
Hierarchical testing: non-inferiority for primary outcome, superiority for
primary outcome, major bleeding, death
ARISTOTLE Efficacy: Apixaban
HR 0.79 (0.66–0.95)
(1.60 %/yr)
21% RRR
(1.27 %/yr )
P (non-inferiority) < 0.001
P (superiority) = 0.011
Granger CB, et al. NEJM 2011; 365:981-992
ARISTOTLE Efficacy Outcomes
Apixaban
(N = 9120)
Outcome
Stroke or systemic embolism*
Warfarin
(N = 9081)
Event
Event Rate
Rate
(%/yr)
(%/yr)
HR (95% CI)
P Value
1.27
1.60
0.79 (0.66, 0.95)
0.011
1.19
1.51
0.79 (0.65, 0.95)
0.012
Ischemic or uncertain
0.97
1.05
0.92 (0.74, 1.13)
0.42
Hemorrhagic
0.24
0.47
0.51 (0.35, 0.75)
< 0.001
0.09
0.10
0.87 (0.44, 1.75)
0.70
All-cause death*
3.52
3.94
0.89 (0.80, 0.998)
0.047
Stroke, SE, or all-cause death
4.49
5.04
0.89 (0.81, 0.98)
0.019
Myocardial infarction
0.53
0.61
0.88 (0.66, 1.17)
0.37
Stroke
Systemic embolism (SE)
Granger CB, et al. NEJM 2011; 365:981-992
ARISTOTLE Safety End Points
Apixaban
(%/yr)
Warfarin
(%/yr)
Hazard Ratio
(95% CI)
Pvalue
ISTH Major Bleeding
2.13
3.09
0.69 (0.60-0.80)
<
0.001
ICH
0.33
0.80
0.42 (0.30-0.58)
<
0.001
GUSTO Severe
0.52
1.13
0.46 (0.35-0.60)
<
0.001
Gastrointestinal
0.76
0.86
0.89 (0.70-1.15)
0.37
Event
Granger CB, et al. NEJM 2011; 365:981-992
ARISTOTLE: Apixaban
Renal Function
Major Bleeding
Annualized Event Rate
Stroke or SEE
Baseline Cockcroft-Gault eGFR mL/min
Hohnloser SH, et al. EHJ 2012 (epub August 29)
Phase III: Protocol Schema
N = 21,105
DOUBLE BLIND
DOUBLE DUMMY
Low dose regimen
Edoxaban 30 mg qd
(n ≈ 7000)
AF on Electrical
Recording < 12 mo
Intended oral A/C
CHADS2 >2
R
Randomization Stratified By
1. CHADS2 2-3 vs 4-6
2. Drug Clearance
High dose regimen
Edoxaban 60 mg qd
(n ≈ 7000)
Active Control
Warfarin
(n ≈ 7000)
Median Duration of Follow-up 24 Months
Primary Objective
Edoxaban: Therapeutically as Good as Warfarin
1º EP = Stroke or SEE (Noninferiority Boundary HR 1.38)
2º EP = Stroke or SEE or CV mortality
Safety EP’s = Major Bleeding, Hepatic Function
SEE = systemic embolic event
EVENT
DRIVEN
Ruff CR et al. Am Heart J 2010; 160:635-41
Pivotal Atrial Fibrillation Trials
Baseline Characteristics
RE-LY
(Dabigatran)
ARISTOTLE
(Apixaban)
ENGAGE AF-TIMI 48*
(Edoxaban)
ROCKET-AF
(Rivaroxaban)
# Enrolled
18,113
18,201
21,105
14,264
Age (yrs)
72 ± 9
70 [63-76]
72 [64-77]
73 [65-78]
Female
36%
35%
38%
40%
CHADS2 score ≥3
32%
30%
52%
87%
VKA naive
50%
43%
41%
38%
Paroxysmal AF
33%
15%
25%
18%
Prior stroke/TIA
20%
19%
18% / 12%
55%**
Diabetes
23%
25%
36%
40%
Prior CHF
32%
35%
56%
62%
Hypertension
79%
87%
90%
91%
*Preliminary data
**includes prior systemic embolism
Connolly SJ et al. N Engl J Med 2009; 361:1139-51
Patel MR et al. N Engl J Med 2011; 365:883-91
Granger CB et al. N Engl J89
Med 2011; 365:981-92
Ruff CR et al. Am Heart J 2010; 160:635-41
Pivotal Atrial Fibrillation Trials
Dose Comparison
Drug
N
Dose (mg)
Frequency
Initial Dose adj*
Dose adj (%)
Dose adj* after
randomization
Design
RE-LY
ROCKET-AF
ARISTOTLE
ENGAGE
AF-TIMI 48
Dabigatran
Rivaroxaban
Apixaban
Edoxaban
18,113
14,266
18,201
21,105
150, 110
bid
20
qd
5
bid
60, 30
qd
No
20 → 15 mg
5 → 2.5 mg
60 → 30 mg
30 → 15 mg
0
21
4.7
> 25
No
No
No
Yes
PROBE
2 x blind
2 x blind
2 x blind
*Dose adjusted in patients with ↓drug clearance.
PROBE = prospective, randomized,
open-label, blinded end point evaluation
Connolly SJ et al. N Engl J Med 2009; 361:1139-51
Patel MR et al. N Engl J Med 2011; 365:883-91
Granger CB et al. N Engl90
J Med 2011; 365:981-92
Ruff CR et al. Am Heart J 2010; 160:635-41
Pivotal Atrial Fibrillation Trials
Results to Date
Drug
Dose (mg)
RE-LY
ROCKET-AF
ARISTOTLE
Dabigatran
110 bid 150 BID
Rivaroxaban
20 mg qd
Apixaban
5 mg bid
Stroke + SEE
non-infer
Superior
ITT cohort: non-infer.
On Rx cohort: Superior
Superior
ICH
Superior
Superior
Superior
Superior
Bleeding
Lower
similar
similar
Lower
Mortality
similar
P = 0.051
similar
Superior: P = 0.047
Ischemic stroke
similar
Lower
similar
similar
Mean TTR
64%
55%
62%
Stopped drug
21%
23%
23%
WD consent
2.3%
8.7%
1.1%
TTR = time in therapeutic range
WD consent = withdrawal of consent, no further data available
Efficacy of New Oral Anticoagulants
Stroke & SEE
Ischemic &
Unsp. Stroke
13%
Hemorraghic
Stroke
55%
Favors NOACs
Miller CS, et al. Am J Cardiol 2012;110(3):453-460.
Favors Warfarin
92
Safety of New Oral Anticoagulants
Bleeding
Major
51%
ICH
GI
Favors NOACs
iller CS, et al. Am J Cardiol 2012;110(3):453-460.
Favors Warfarin
Does INR Matter?
ROCKET AF
0.00-50.62%
50.71-58.54%
58.63-65.71%
65.74-100.0%
Treatment Group
Warfarin
P-value
Event Rate / Year Event Rate / Year (interaction)
0.74
1.77
2.53
1.94
2.18
1.90
2.14
1.33
1.80
RE-LY (Dabigatran 150 mg)
< 57.1%
1.1
57.1–65.5%
1.04
65.5–72.6%
1.04
> 72.6%
1.27
Hazard Ratio (95% CI)
Study Drug
Favors
Warfarin
0.20
1.92
2.06
1.51
1.34
ARISTOTLE
< 58.0%
58.0–65.7%
0.29
1.75
1.30
2.28
1.61
65.7–72.2 %
> 72.2 %
1.21
0.83
1.55
1.02
Wallentin L, et al. Lancet 2010;376:975-983
Patel, et al. NEJM 2011;365(10);883-891
Granger CB, et al. NEJM 2011; 365:981-992
0.2
1
www.fda.gov
2
5
All-Cause Death &
Thromboembolism
Warfarin Treatment Interruption
Raunsø J, et al. Eur Heart J 2012; 33:1886-1892
Novel Oral Anticoagulants
More Events “Off-Drug”
13%
25%
%/yr
P = 0.015
P = 0.117
ROCKET AF
Rivaroxaban Increased Events at End of Trial
81.3
# Primary Events
Warfarin
P = 0.008
48.8
Rivaroxaban
Rivaroxaban
Warfarin
# Primary Events during first 30 days of transition
Safety/Days 3 to 30 after the last dose
Patel MR, et al. NEJM 2011; 365:883-891
Piccini JP, AHA Emerging Science Series, April 25, 2012 webinar. Abstract 114
ARISTOTLE
Apixaban Increased Events at End of Trial
Days after
last dose
Apixaban to VKA group
n/N
Warfarin to VKA group
%/year
n/N
%/year
Stroke or systemic embolism
1–30
21/6791
4.02
5/6569
0.99
1–2
1/6791
2.69
1/6569
2.78
3–7
4/6787
4.31
0/6566
0
8–14
5/6780
3.85
1/6559
0.80
15–30
11/6771
4.18
3/6548
1.18
Pattern mirrored the first 30 days of the trial where warfarin-naïve patients starting
warfarin had a higher rate of stroke or systemic embolism (5.41%/year) than
warfarin-experienced patients (1.41%/year).
Granger CB, et al. European Heart Journal 2012; 23 (Supplement):685-686
“After the Deluge” of SPAF Trials
Translating Trials into Practice and
Guidelines: 2012 Update
Post-Trial, Real World Concerns, Guidelines,
and Actions—Where Have Landmark SPAF
Trials Taken Us? How Have Recent Guidelines
Made Sense of These Data?
NOACs Elevated to "Favored" Status by ESC
2012 Update Guidelines for
Management of AF
The net clinical benefit of VKAs, balancing ischaemic
stroke against ICH in patients with non-valvular AF, has been
modeled on to stroke and bleeding rates from the Danish
nationwide cohort study for dabigatran, rivaroxaban, and
apixaban, on the basis of recent clinical trial outcome data
for these NOACs. At a CHA2DS2-VASc score of 1, apixaban
and both doses of dabigatran (110 mg bid and 150 mg bid)
had a positive net clinical benefit while, in patients with
CHA2DS2-VASc score ≥ 2, all three NOACs were superior to
warfarin, with a positive net clinical benefit, irrespective of
bleeding risk.
2012 focused update of the ESC Guidelines for the management of atrial fibrillation: Europace. 2012 Aug 24
Assess bleeding risk
(HAS-BLED score)
Consider patient values
and preferences
NOAC
VKA
LINE SOLID = BEST OPTION
DASHED = ALTERNATIVE OPTION
2012 focused update of the ESC Guidelines for the management of atrial fibrillation: Europace. 2012 Aug 24
Bleeding Risk with Dabigatran
Fact vs Fiction
Bleeding Risk with Dabigatran
Fact vs Fiction: What Do Regulators Conclude?
Disconnect Between Clinical Trials and Post-Marketing
Surveillance Bias: A Case Study
EMA Report on Dabigatran: May 24, 2012
"What are the conclusions of the CHMP?
The CHMP concluded that the latest available data are
consistent with the known risk of bleeding and that the risk
profile of dabigatran was unchanged. The Committee
found that frequency of reported fatal bleedings with
dabigatran was significantly lower than what had been
observed in clinical trials at the time of authorisation, but
considered that the risks should nonetheless continue to
be kept under close review."
EMA Report on Dabigatran
May 24, 2012
What is the updated advice for prescribers?
Prescribers are reminded of the need to follow all the necessary
precautions with regard to the risk of bleeding with dabigatran,
including the assessment of kidney function before treatment in all
patients and during treatment if a deterioration is suspected, as well
as dose reductions in certain patients.
Dabigatran must not be used in patients with a lesion or condition
putting them at significant risk of major bleeding (see the revised
product information for details).
Dabigatran must not be used in patients using any other
anticoagulant, unless the patient is being switched to or from
dabigatran (see the revised product information for details).
A European Commission decision on this opinion will be issued in
due course.
Dabigatran vs. Warfarin: Surgical
Bleeding
, even
among patients having major or urgent
surgery. Patients receiving dabigatran were 4
times more likely to have their procedure or
surgery within 48 hours of withholding
anticoagulation
Circulation, Healey et al., 2012
ESC 2012 Atrial Fibrillation
Guidelines Update: Risk Assessment
Risk Profile
Class / Level
CHA2DS2-VASc = 0
No antithrombotic therapy
IB
CHA2DS2-VASc = 1
VKA (INR 2-3)
Or
Dabigatran / Rivaroxaban / Apixaban
IIa A (Favored)
CHA2DS2-VASc ≥ 2
VKA (INR 2-3)
Or
Dabigatran / Rivaroxaban / Apixaban
I A (Favored)
Conclusions: From Trials and
Evidence to Strategy and Practice
►
New therapies provide the promise of providing safer,
more effective, and more convenient anticoagulation.
Trials are consistent in reduction of intracranial
hemorrhage and bleeding mortality.
►
SPAF trials are consistent in demonstrating that NOACs
are at least as good as, and in some cases, superior to
warfarin in preventing stroke in patients with AF.
►
There are important differences in the PK/PD of these
agents (half-life, metabolism, renal elimination) that will
alter the risk/benefit profile in specific populations; in
particular, careful monitoring of renal function is a
precondition for optimizing safety and efficacy of these
agents.
Conclusions
►
No reversal agent is currently available but whether the
lack of such agents lead to increased bleeding or
mortality risk has not been substantiated.
►
New ESC 2012 Update Guidelines for AF have refined
and incorporated a new suite of risk prediction
strategies (CHA2DS2-VASc, HAS-BLED) that will result in
a greater proportion of patients being eligible for oral
anticoagulation.
►
New ESC 2012 Update Guidelines for AF have elevated
NOACs to "favored" status over VKA for patients who
meet risk stratification criteria for requiring oral
anticoagulation for AF.
New Paradigms in the
Science and Medicine of Heart Disease
Stroke Prevention in
Atrial Fibrillation
Megatrends, Challenges, and
Clinical Dilemmas
Samuel Z. Goldhaber, MD, Program Chairman
Director, VTE Research Group
Cardiovascular Division
Brigham and Women’s Hospital
Professor of Medicine
Harvard Medical School
Faculty COI Disclosures
Research Support
Eisai, EKOS, Johnson & Johnson, sanofi-aventis
Consultant
Baxter, Boehringer-Ingelheim, Bristol-Myers
Squibb, Daiichi, Eisai, Janssen, Merck, Pfizer,
Portola, sanofi-aventis
Risk Assessment Megatrend
►
CHA2DS2-VASc has replaced CHADS2 as the
predominant assessment tool to predict stroke
risk (ESC 2012 AF Guidelines Update).
►
HAS-BLED has gained dominance as the most
predictive bleeding index. It is best used as a
cautionary “yellow flag” rather than as a reason
to withhold anticoagulation (ESC 2012).
Clinical Dilemma and Challenge—
Stroke Risk Underestimated
►
Paroxysmal AF is difficult to detect.
►
24h Holter is often insufficient. Long-term
noninvasive or invasive monitoring may be
necessary.
►
Many strokes are misclassified as “cryptogenic”
and are treated with aspirin or other antiplatelet
agents, with questionable efficacy for AF.
►
The misclassified strokes are really
thromboembolic and warrant anticoagulants.
Subclinical AF and Risk of Stroke
Stroke or Systemic Embolism
Atrial tachyarrhythmia > 6 min ≤ 3 months after
pacemaker or defibrillator implantation
Healey JS, et al. NEJM 2012; 366:120-129
Years
Redefining Risk vs Benefit for OAC
HAS-BLED
Letter
Clinical
Characteristic
Points
H
Hypertension
1
Stroke
(% / yr)
A
Abnormal Liver
or Renal
Function
HAS-BLED
Score
1 or 2
0
1.1 %
1
1.0 %
S
Stroke
1
2
1.9 %
B
Bleeding
1
3
3.7 %
L
Labile INR
1
4
8.7 %
E
Elderly
(age > 65)
1
>5
?? %
Lip GYH. Am J Med. 2011;124:111-114.
D
Drugs or Alcohol
Maximum Score
1 or 2
9
ESC Guidelines: Eur Heart J . 2010;31:2369-2429.
Clinical Dilemma: Bleeding Risk
Correlates With Stroke Risk
►
The higher the bleeding risk, as assessed by the
HAS-BLED Index, the higher the stroke risk—A
“Catch 22” when considering and/or deploying
oral anticoagulation.
►
Based on observational and trial evidence, we
must be especially vigilant to prescribe
anticoagulation to AF patients at high risk of
bleeding, when the thrombosis risk assessment
justifies this course of action.
Action Plan When OAC is Indicated
and Patient Has High HAS-BLED Index
►
Modify bleeding risk factors.
►
Intensify surveillance for bleeding and for
triggers that cause bleeding.
►
Consider “renal dose” for NOAC, especially in the
presence of some renal dysfunction or frailty or
age ≥ 80 years.
►
Monitor renal function with vigilance.
►
Prescribe PPI when indicated.
Stroke and Bleeding in Atrial Fibrillation
with Chronic Kidney Disease
Danish
Registry
146,251
patients were
discharged with
nonvalvular atrial fibrillation (1997-2008)
13,879 were excluded
127,884 (96.6%) did
not have renal disease
3587 (2.7%) received a
diagnosis of non-end-stage
chronic kidney disease
4538 (3.5%) received a
diagnosis of non-end-stage
chronic kidney disease
Olesen JB. NEJM 2012; 367: 625-635
228 (6.4%) underwent
renal-replacement therapy
during follow-up
901 (0.7%) underwent
renal-replacement
therapy
Stroke Risk and Renal Disease
Aspirin does not prevent stroke
Characteristic
Total Population
(n = 132,372)
Hazard Ratio
(95% CI)
P Value
All participants
No Renal Disease
(n = 127,884)
Hazard Ratio
(95% CI)
P Value
1.00
Antithrombotic
Therapy
None
1.00
1.00
Warfarin
0.59 (0.57-0.62)
< 0.001
1.10 (1.06-1.14)
< 0.001
Aspirin
1.11 (1.07-1.15)
< 0.001
1.10(1.06-1.14)
< 0.001
Warfarin and
aspirin
0.70(0.65-0.75)
< 0.001
0.69(0.64-0.74)
< 0.001
Olesen JB. NEJM 2012; 367:625-635
Bleeding Risk and Renal Disease
Aspirin and warfarin/aspirin increase bleeding
Characteristic
Total Population
(n = 132,372)
Hazard Ratio
(95% CI)
P Value
All participants
No Renal Disease
(n = 127,884)
Hazard Ratio
(95% CI)
P Value
1.00
Antithrombotic
Therapy
None
1.00
1.00
Warfarin
1.28(1.23-1.33)
< 0.001
1.28(1.23-1.33)
< 0.001
Aspirin
1.21(1.16-1.26)
< 0.001
1.21(1.16-1.26)
< 0.001
Warfarin and
aspirin
2.15(2.04-2.26)
< 0.001
2.18(2.07-2.30)
< 0.001
Olesen JB. NEJM 2012; 367:625-635
Megatrend:
Recognizing Overuse of Aspirin
Role of aspirin in the setting of
SPAF is called into question.
Aspirin is often prescribed for “CAD prevention,”
without a clear evidence-based rationale, thus
increasing bleeding risk when combined with OACs
used for SPAF. Evaluate necessity for ASA.
Dosing Options for Renal Dysfunction
Consider also for age ≥80, weight ≤ 60 KG (frailty)
►
Dabigatran 75 mg bid (USA)
►
Dabigatran 110 mg bid (non-USA)
►
Rivaroxaban 15 mg daily
25%
►
Apixaban 2.5 mg bid
50%
ESC 2012
50%
Dilemmas in “Under-Anticoagulation”
►
Anticoagulants clearly prevent stroke in AF
patients but are markedly underutilized
►
Failure to prophylax in the setting of nonvalvular AF is characterized by fear of:
●
●
●
●
Bleeding
Older age
Renal dysfunction
Lack of medication adherence
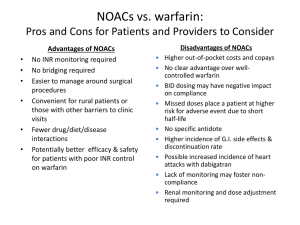
Dilemmas: NOACs vs Warfarin
►
By most metrics, NOACs are the “best option”
for SPAF (ESC 2012 Update for AF)
►
Failure to prescribe NOACs is characterized by:
●
●
●
●
Lack of familiarity
Lack of reversal agent
Inability to measure NOAC level
Inertia, fear of change, “preapprovals”
NOACs vs Warfarin—
A View From 30,000 Feet
►
NOACs generally more effective than warfarin
for stroke prevention
►
NOACs are generally safer (less bleeding, with
some exceptions, but NOACs uniformly cause
less intracranial hemorrhage, most devastating
and mortality-inducing bleeding complication of
OAC)
►
NOACs, overall, reduce mortality
►
NOACs are more convenient for patient/clinician
New Anticoagulant Therapies Compared
to Warfarin: All-cause Mortality
Dabigatran 150 mg b.i.d.
Dabigatran 110 mg b.i.d.
Rivaroxaban 20 mg o.d.
Abixaban 5 mg b.i.d.
0.5
Connolly S et al NEJM 2009; Patel M et al NEJM
2011; Granger CB et al NEJM 2011
1
2
Limitations of Novel Agents are
Exaggerated
No antidote when bleeding
• Best treatment is prevention
• Warfarin has no great antidote
• Time is a great antidote
No antidote for urgent procedures
• RELY analysis 2012 shows no
increase in bleeding in this
setting
Lack standard measurement
• Do not need one
• Time since last dose is helpful
Dependence on renal function
• Rivaroxaban, apixaban modest
renal effect
Cost
• Highly cost effective in analyses
Deciphering the Pharmaco-economic Maze
“Cost-effectiveness”
Cost: Must take into account the costs of
caring long-term for debilitated
thromboembolic stroke patients and the costs
of caring for intracranial hemorrhage when
doing a “cost-effectiveness” analysis of
NOACs vs warfarin.
However, we continue to have mostly
“silo budgeting.”
Cost of Dabigatran vs Warfarin
► Dabigatran retail: $240/month
► Warfarin discount retail: $4/month
► Will the high price of dabigatran cause poor
medication adherence?
“The cost of medical care looms as the single largest
threat to the US economy.”
Avorn J. Circulation 2011: 123: 2519-2521
Prevent > 50% AF Cases by Modifying
Cardiovascular Risk Factors
►
N = 14,598 middle-aged subjects
►
Over 17 years, 1520 incident cases of AF in
the Atherosclerosis Risk in Communities
(ARIC) Study
►
56% of cases explained by elevated CV risk
factors, especially hypertension, obesity,
diabetes, and smoking
Circulation 2011; 123: 1501-1508
Guidelines for “Bridging”with
Dabigatran (RE-LY)
Renal Function
Impairment
(CrCL mL/min)
Estimated Halflife, h (Range)
Stopping Dabigatran Before
Surgery/Procedure
High Risk for
Bleeding
Standard Risk
for Bleeding
Mild:
> 50 to 80
15 (12-18)
2-3d*
24 h (2 doses)
Moderate:
> 30 to < 50
18 (18-24)
4d
At least 2 d
(48 h)
Severe: < 30
27 (> 24)
> 5d
2-4 d
Healey JS. Circulation 2012; 126: 343-348
Interrupting Dabigatran and Warfarin
RE-LY
►
1 of 4 patients underwent peri-procedural
anticoagulant interruption
►
Stroke rate: 0.5%; major bleeding rate:
4%, 7 days pre- to 30 days post
►
Dabigatran was withheld an average of 2
days, whereas warfarin was withheld an
average of 5 days preop
Healey JS. Circulation 2012; 126: 343-348
Medication Adherence Failure
►
Failing to fill or refill a prescription
►
Omitting doses
►
Overdosing
►
Prematurely discontinuing medication
►
Taking someone else’s medication
►
Taking a medication with prohibited foods
►
Taking outdated medications
Questions Regarding the New
Non-Monitored, Oral Anticoagulants
►
Do they represent a significant improvement
for patients who have been taking warfarin
with consistently therapeutic INR values for months
or years? They may.
►
Will the elimination of regular INR measurement
reduce or improve compliance?
►
How will their cost compare to current costs
(including INR monitoring, dose adjustment, etc)?
Selecting Patients for Non-Monitored
Oral Anticoagulation (NOAC)
Clinical Dilemma #1
►
Which patients are the best candidates for nonmonitored oral anticoagulation? Treatment-naive and de
novo patients? And/or patients on warfarin?
►
Established patients on warfarin doing "well?"
Established on warfarin, doing well, but at high risk for
bleeding? High HAS-BLED? Previous stroke?
►
On warfarin, and doing "reasonably" well, but requiring
multiple interventions to keep INR/TTR controlled?
►
Patients on warfarin who are doing well, but only with
intensive monitoring?
Transitioning Patients from Warfarin to a
Non-Monitored Oral Anticoagulant
Clinical Dilemma #2
►
How do we actually transition patients from
warfarin to dabigatran, rivaroxaban, apixaban, or
other agents?
►
What INR do we wait for?
►
What are the renal issues that need to be
considered for each agent?
How do I Convert a Patient from
Warfarin to Dabigatran and Vice Versa?
► Warfarin to dabigatran: Discontinue warfarin and start
dabigatran when the international normalized ratio
(INR) is below 2.0.
► Dabigatran to warfarin:
• CrCl > 50 mL/min, start warfarin 3 days before discontinuing dabigatran.
• CrCl 31-50 mL/min, start warfarin 2 days before discontinuing dabigatran.
• CrCl 15-30 mL/min, start warfarin 1 day before discontinuing dabigatran.
• CrCl < 15 mL/min, no recommendations can be made.
Because dabigatran can contribute to an elevated INR, the INR
will better reflect warfarin’s effect after dabigatran has been
stopped for at least 2 days.
Dabigatran prescribing information 2010
Managing Patients Who Are on Non-Monitored
Oral Anticoagulation and Have Had a Stroke
Clinical Dilemma #3
►
How do we approach a patient who has had an
embolic stroke while on a non-monitored oral
anticoagulant?
►
Should we switch? Why? Why not?
►
To what agent would we switch? From one nonmonitored oral anticoagulant to another? To warfarin?
►
If we switch to warfarin, at what INR? What if the
patient is at risk for hemorrhage?
What Should I Do if my Patient Has
an Ischemic Stroke on Dabigatran?
►
Consider:
●
●
●
●
Is the patient compliant with dabigatran? Check
aPTT or thrombin time– if dose taken within past
12 hours, these levels should be prolonged.
If the stroke is cryptogenic, consider adding
antiplatelet therapy.
Convert dabigatran to warfarin (target INR 2-3 or
higher?).
Switch to another NOAC?
Aligning Specific Patient and Risk Profiles with
Specific NOACS: Apixaban, Dabigatran, Rivaroxaban
Clinical Dilemma #4
►
Based on AVERROES, RE-LY, ARISTOTLE, and ROCKETAF, should we be aligning specific, non-monitored oral
anticoagulants with specific risk groups?
►
Should the warfarin-intolerant/ineligible patient be
"steered" toward apixaban?
►
The "high-risk" patient be steered toward rivaroxaban?
►
The intermediate-risk patient be "steered" toward
apixaban or dabigatran?
►
How do we know whether this kind of alignment is
evidence-based, or if it is an artifact of the trial designs?
What Should We Use For Hemorrhage on a NonMonitored Oral Anticoagulant?
Clinical Dilemma #5
►
What should be our clinical approach to a patient
who has had a hemorrhage on a non-monitored
oral anticoagulant?
►
Does it depend on the type of hemorrhage?
Other factors?
►
Should we ever consider warfarin in these
patients?
Guide to the Management of
Bleeding in Patients Taking NOAC
Patients with bleeding on NOAC therapy
Moderate-Severe
bleeding
Mild bleeding
• Delay next dose or
discontinue treatment
as appropriate
•
•
•
•
•
•
•
Mechanical compression
Surgical intervention
Fluid replacement and
hemodynamic support
Blood product transfusion
Oral charcoal
Hemodialysis
? Prothrombin Complex
Concentrate?
Life-threatening
bleeding
• Consideration of rFVIIa
or PCC
• Charcoal filtration
• ? Prothrombin Complex
Concentrate
(Circulation 2011; 2011: 124:
1573-9)
(Circulation 2011; 2011: 124: 1573-9)
Hankey GJ and Eikelboom JW. Circulation. 2011; 123: 1436-1450
Antithrombotic Agents
A New Era of “Alignment and Flexibility?”
►
Dabigatran: Superior SPAF compared with warfarin
►
Rivaroxaban: Once-daily administration and less
dependence on kidneys for metabolism; non-inferior in
ITT analysis in very high-risk patient population
►
Apixaban: Safety equivalent to aspirin in AVERROES,
and superior stroke prevention in warfarin intolerant or
ineligible
►
Apixaban: Superior SPAF, less major bleeding, lower
all-cause mortality.
SPAF Clinical Trial Programs
Translational Dilemmas and Cautionary Notes
►
Do clinical trial results apply to “real world”
medicine in busy clinical practices with brief office
visits and minimal telephone follow-up?
►
Are patients who participate in clinical trials
healthier/more motivated than most? Does this
make favorable results more likely in both the new
drug and the comparison groups?
►
Do the costs of a “copay” affect patient decisions
to fill a prescription for a potentially more effective,
safer drug vs a less expensive but less effective
alternative?
Unresolved Issues with NOACs
►
No established methods of monitoring
►
No known therapeutic ranges
►
Lack of a proven antidote
►
Uncertain management of bleeding
►
Long term safety: to be determined
►
No head-to-head comparisons of new agents
Properties of Ideal Anticoagulant
Do NOACS Fit the Bill?
►
Proven efficacy √
►
Low bleeding risk √
►
Fixed dosing √
►
Good oral bioavailability √
►
No routine monitoring needed √
►
Reversibility: ?PCC, FEIBA, rVIIa
►
Rapid onset of action √
►
Few drug or food interactions √
NOACs: Advancing Opportunities
to Connect Guidelines with Practice
►
Lower stroke rates
►
Fewer major and fatal bleeds, especially ICH
►
Lower dose options for chronic kidney disease,
elderly, and the “frail” or “underweight” patient
►
Use in conjunction with RF reduction: treat
congestive heart failure, diabetes, hypertension,
obesity
►
Facilitate periprocedural treatment
►
Should improve medication adherence—no
injections/ no routine lab blood testing
Advances in the Science and Medicine
of Stroke Prevention in AF
So What Now? Trials in Translation
Applying Evidence-Driven Strategies to AF
Patients at the Front Lines of Clinical Practice
Audience Response System-Based Interactions
Samuel Z. Goldhaber, MD
Program Chairman
Director, VTE Research Group
Cardiovascular Division
Brigham and Women’s Hospital
Professor of Medicine
Harvard Medical School
Atrial Fibrillation Case Study #1
► A 71-year-old white female with a history of
chronic, non-valvular AF, controlled hypertension,
and a history of mild congestive heart failure has
been evaluated by a cardiologist and found to be
a suitable candidate for warfarin therapy.
► Due to logistical barriers that make monitoring
difficult and dietary variations, the patient has
had difficulty controlling her INR.
► Wide fluctuation in her INR has made her
question continued warfarin therapy.
Audience Response System (ARS) Question
► Because of her high risk for embolic stroke, her
cardiologist is considering alternative forms of
thromboprophylaxis for SPAF. She has a HAS-BLED
SCORE of 2.
► Which of the following should we consider? Are any
of these strategies optimal in this patient type?
1) Keep patient on warfarin
2) Replace warfarin with aspirin
3) Replace warfarin with aspirin + clopidogrel
4) Replace warfarin with a non-monitored oral anticoagulant
Atrial Fibrillation Case Study #2
► An 81-year-old white female with a history of chronic,
non-valvular AF, a history of a previous ischemic
stroke, and a history of mild congestive heart failure
has been on a combination of clopidogrel and aspirin
therapy because she was found to be intolerant of
warfarin.
► She is on a proton pump blocker, an ACE inhibitor, a
diuretic, and digoxin.
► She is admitted to the hospital for a GI bleed, and is
found to have a hematocrit of 29 and a hemoglobin
of 9.8. The aspirin and clopidogrel are discontinued.
Atrial Fibrillation Case Study #2
The patient stabilizes, and the cardiologist is
consulted to determine the subsequent course of her
antithrombotic treatment. She has a HAS-BLED score
of 3.
It is your opinion that:
1) Because of the documented GI bleed, the patient should
not be treated with antithrombotic agents, because the
risk of bleeding outweighs the risk of stroke and its
complications.
2) Because of the patient's risk profile, there should be an
attempt to provide thromboprophylaxis against embolic
stroke.
Atrial Fibrillation Case Study #2
The cardiologist has determined that this
patient requires antithrombotic management
for stroke prevention.
At this point you would most likely:
1) Try the patient on warfarin again
2) Try to re-introduce clopidogrel and aspirin
3) Treat the patient with aspirin alone
4) Introduce a non-monitored oral anticoagulant to
the patient's regimen.
Atrial Fibrillation Case Study #3
► An 82-year-old man with hypertension and
diabetes has permanent atrial fibrillation.
► He has a history of spinal stenosis and walks
with a walker and has a history of falls.
► He has a CHADS-VASc score of 3, and a HAS—
BLED score of 2.
► Which regimen would you prescribe for
prophylaxis against thromboembolism?
Atrial Fibrillation Case Study #3
Which regimen would you prescribe for
prophylaxis against thromboembolism?
1. Warfarin (INR 2.0-3.0)
2. Warfarin (INR 1.5-2.0)
3. Aspirin 81 mg daily
4. Aspirin 81 mg + clopidogrel 75 mg daily
5. An oral Factor Xa or direct thrombin inhibitor
Atrial Fibrillation Case Study
Anticoagulation in Patients at Risk of Falls
“…persons taking warfarin must fall about
295 (535/1.81) times in 1 year for
warfarin not to be the optimal therapy…”
Atrial Fibrillation Case Study #4
A 71-year-old man with AF, heart failure, and a prior
history of stroke presents with unstable angina and
proceeds to cardiac catheterization where a culprit
lesion is identified. Optimal management includes:
1) Placement of a drug-eluting stent with plan to continue
anticoagulation in addition to 1 year of dual antiplatelet
therapy
2) Placement of a drug-eluting stent with 1 year of dual
antiplatelet therapy alone
3) Placement of a bare metal stent with plan to continue
anticoagulation in addition to 1 month of dual antiplatelet
therapy
4) Placement of a bare metal stent with 1 month of dual
antiplatelet therapy alone
Atrial Fibrillation Case Study #5
A 67-year-old female with a history of mitral
stenosis with subsequent mechanical mitral valve
replacement has AF.
Which of the following anticoagulants can be used
for stroke prevention in this patient?
1) Warfarin
2) Dabigatran
3) Apixaban
4) Rivaroxaban
5) All of the above
Atrial Fibrillation
Knowledge Assessment Question
The major potential benefits of the new nonmonitored oral anticoagulants include:
1) Rapid therapeutic anticoagulant effect
2) Greater safety with regards to intracranial hemorrhage
3) Proven reversal agent
4) All of the above
5) Both 1 and 2
Atrial Fibrillation Case Study #6
An 82-year-old man with AF has had several admissions
over the past 6 months for heart failure complicated by
worsening renal function. His creatinine clearance is
currently 20 mL/min but frequently fluctuates to 10-15
mL/min. He has a HAS-BLED score of 3.
The best anticoagulant regimen for stroke prevention is:
1) Dabigatran 150 mg twice daily
2) Dabigatran 75 mg twice daily
3) Warfarin titrated to goal INR 2-3
4) Rivaroxaban 20 mg once daily
5) Rivaroxaban 15 mg once daily
Atrial Fibrillation Case Study #7
A 79-year-old woman with a CHADS-VASc score of
2 who has been on warfarin for the past 2 years
returns to clinic for routine follow-up.
Her INR control has been excellent and she has
never experienced a stroke or had significant
bleeding. Her HAS-BLED score is 2.
Her complaints today are thinning hair, cold
intolerance, and fatigue.
Her laboratory work is normal including a TSH.
Atrial Fibrillation Case Study #7
Which of her symptoms could be due to warfarin?
1) Thinning hair
2) Cold intolerance
3) Fatigue
4) Both 1 and 2
5) All of the above
Atrial Fibrillation Case Study #8
A 69-year-old woman with AF and CHADS2 score
of 4 has a creatinine clearance that is stable at 40
mL/min.
Which of the following anticoagulation regimens
are suitable for her?
1) Dabigatran 150 mg twice daily
2) Dabigatran 75 mg twice daily
3) Rivaroxaban 20 mg once daily
4) Rivaroxaban 15 mg once daily
5) Both 1 and 4
Atrial Fibrillation Case Study #8
What would her options be if her creatinine
clearance was stable at 25 mL/min?
1) Dabigatran 75 mg twice daily
2) Rivaroxaban 15 mg once daily
3) Only warfarin can be used in patients with creatinine
clearance < 30 mL/min
4) Both 1 and 2
Atrial Fibrillation Case Study #9
A 74-year-old man with AF on dabigatran is
involved in a motor vehicle accident and needs
emergency surgery.
It is unclear if he is taking this medication but
the surgeon is concerned about operating on
him if he is fully anticoagulated.
Atrial Fibrillation Case Study #9
Which of the following lab tests, if normal, would
reassure the team that the patient is not
anticoagulated?
1) INR (international normalized ratio)
2) aPTT (activated partial thromboplastin time)
3) PT (prothrombin time)
4) Bleeding time
Atrial Fibrillation Case Study #10
A 60-year-old man with AF has been on warfarin
but it has been very difficult to control his INR.
You have decided to switch to dabigatran. Which
of the following is true regarding transitioning a
patient from warfarin to dagibatran?
1) Start dabigatran when his INR < 3
2) Start dabigatran when his INR < 2
3) Start dabigatran 24 hours after his last dose of warfarin
Atrial Fibrillation Case Study #10
What if you decided to switch the patient to
rivaroxaban?
1) Start rivaroxaban when his INR < 3
2) Start rivaroxaban when his INR < 2
3) Start rivaroxaban 24 hours after his last dose of
warfarin
Atrial Fibrillation Case Study #11
A 78-year-old female with AF, systolic heart failure,
hypertension, diabetes, and a history of significant GI
bleeding has been on warfarin for many years but has
had a difficult time controlling her INR with frequent
supertherapeutic values despite intensive monitoring
and titration of her warfarin dose. Her HAS-BLED
score is 3. The best treatment option for her is:
1) No antithrombotic therapy
2) Discontinue warfarin and start aspirin
3) Discontinue warfarin and start dabigatran
4) Discontinue warfarin and start rivaroxaban
5) Discontinue warfarin and start apixaban
Atrial Fibrillation Case Study #12
A 76-year-old woman with heart failure, hypertension,
diabetes, and declining renal function (creatinine
clearance 35 mL/min) has an embolic stroke due to
newly diagnosed AF. She refuses to take warfarin.
What is the best validated antithrombotic regimen in
this particular patient?
1) Aspirin
2) Aspirin and clopidogrel
3) Dabigatran
4) Apixaban
5) Rivaroxaban
Atrial Fibrillation Case Study #13
A 68-year-old man with a mechanical mitral valve
develops AF.
The best anticoagulant option for him is:
1) Warfarin
2) Dabigatran
3) Apixaban
4) Rivaroxaban
5) Aspirin
Atrial Fibrillation Case Study #14
A 76-year-old man with heart failure and hypertension
undergoes successful catheter ablation for
symptomatic AF.
Which of the following is true regarding his
anticoagulation management?
1) He no longer requires anticoagulation now that he is in
sinus rhythm
2) Patient should be on both aspirin and an anticoagulant
3) Patient should be on an anticoagulant alone
4) Aspirin and clopidogrel together is as effective as
anticoagulation in these patients
Atrial Fibrillation Case Study #14
The cardiologist has determined that this
patient requires antithrombotic management for
stroke prevention. At this point you would most
likely:
1) Try the patient on warfarin again
2) Treat the patient with aspirin alone
3) Introduce the non-monitored oral anticoagulant,
apixaban, into the patient's regimen
4) Introduce dabigatran into the patient’s regimen
5) Introduce rivaroxaban into the patient’s regimen
Atrial Fibrillation Case Study #15
► A 75-year-old male with a history of chronic,
non-valvular AF, diabetic renal disease,
previous history of ischemic stroke, history of
mild HF, and controlled hypertension has been
on warfarin therapy. The HAS-BLED score is 4.
► For the past 6 months, despite repeated visits
for monitoring and warfarin dose adjustment,
his INR has varied between 1.5 and 4.3.
► His estimated GFR is 30 mL/min.
Atrial Fibrillation Case Study #15
At this point you would:
1) Continue to try to stabilize his INR on warfarin
2) Change to aspirin alone
3) Introduce the non-monitored oral anticoagulant rivaroxaban
into the patient's regimen
4) Introduce the non-monitored oral anticoagulant apixaban
into the patient's regimen
5) Introduce the non-monitored oral anticoagulant dabigatran
into the patient's regimen
Atrial Fibrillation Case Study #17
► An 82-year-old man with hypertension,
diabetes, mild congestive heart failure, and
previous ischemic stroke, is diagnosed with
atrial fibrillation.
► He has not been taking any anticoagulants.
Atrial Fibrillation Case Study #17
Which regimen would you initiate for prophylaxis
against stroke?
1) Warfarin (INR 2.0-3.0)
2) Aspirin 81 mg + clopidogrel 75 mg daily
3) Rivaroxaban
4) Apixaban
5) Dabigatran
Atrial Fibrillation Case Study #18
► An 82-year-old man with hypertension,
diabetes, mild CHF, and a previous
ischemic stroke has permanent atrial
fibrillation.
► He has been on warfarin for about 5
years and his INR has remained constant
between 2.3 and 2.7.
► He has a HAS-BLED score of 3.
Atrial Fibrillation Case Study #18
Which regimen would you continue or switch to for
prophylaxis against stroke?
1) Continue current therapy with warfarin
2) Aspirin 81 mg + clopidogrel 75 mg daily
3) Rivaroxaban
4) Apixaban
5) Dabigatran
Atrial Fibrillation Case Study #19
A 75-year-old man with a CHADS2 of 3 has been
taking dabigatran 150 mg for SPAF. His estimated
GFR was 55 mL/min 6 months ago and is now 40
mL/min.
I would now:
1) Continue to monitor patient
2) Switch patient to 75 mg dabigatran twice per day
3) Switch patient to warfarin
4) Switch patient to rivaroxaban
5) Start ASA and clopidogrel
New Paradigms in the
Science and Medicine of Heart Disease
Interactive Question
and Answer Session