Intermediate risk - The Prostate Net
advertisement

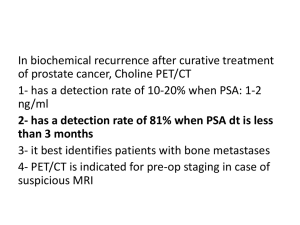
Radiation Therapy for Treatment of Prostate Cancer Stephen Ko, M.D. Mayo Clinic Florida August 30, 2010 Overview I. U.S. Epidemiology II. Types of Radiation III. Anatomy IV. Technologic Advances – 2-Dimensional Planning – Intensity Modulated Radiotherapy – Brachytherapy V. Definition of Risk Categories – Low Risk – Intermediate Risk – High Risk Overview VI. Dose-escalation Trials – – – – MSKCC IMRT Dose Escalation Proton Beam Dose Escalation MDACC Randomized Trial (70 Gy vs. 78 Gy) Harvard Randomized Trial (70.2 GyE vs. 79.2 GyE) VII. Low Risk Disease Treatment – IMRT alone – Seeds alone VIII. Intermediate Risk Disease Treatment – IMRT alone – 6 mo Hormone + EBRT – Seeds + EBRT Overview IX. High Risk Disease Treatment – Long-term Hormonal therapy Randomized Trials RTOG randomized Trial EORTC randomized Trial – Seeds + EBRT X. Comparing Modalities (Surgery vs. Radiation) XI. Quality of Life Comparison XII. Conclusions I. U.S. Epidemiology 2009 New cases prostate cancer: 192,280 Deaths from prostate cancer: 27,360 New cases prostate cancer in FL: 12,380 New cases prostate cancer in GA: 5,210 Death from prostate cancer in FL: 2,470 Death from prostate cancer in GA: 870 U.S. Epidemiology 2009 New Cases in U.S. U.S. Epidemiology . 2009 Deaths/year from cancer in U.S. II. Types of Radiation • External Beam: high energy X-rays given with linear accelerator • • Primary therapy Postoperative • Brachytherapy: radioactive seeds Primary therapy After external: boost dose • Proton Beam: heavy particle • Primary therapy What is dose? Dose is the amount of radiation used to treat a patient SI unit (joules/kg) Gray (Gy) Centigray (cGy) 100 cGy = 1 Gy Similar to milligrams for drugs 180 cGy or 200 cGy per day or 1.8 Gy or 2 Gy per day is usually given to treat prostate 1.8 Gy x 42 treatments = 75.6 Gy total III. Anatomy Seminal vesicles Bladder Rectum Prostate IV. Technological Advances 2-Dimensional Planning (Fluoroscopicbased) 3-Dimensional Planning (CT-based) Intensity Modulated Radiotherapy or IMRT (CT-based) 2- Dimensional Vs. 3-Dimensional Planning Rectum Bladder Prostate Prostate Rectum External Beam Electronic Portal Imaging Intraprostatic Marker Localization CT Scan Intended treatment X-ray on the machine Actual treatment Gold marker Final position Initial setup Positional error corrected Intensity Modulated External Beam Radiotherapy IMRT Prostate Dose Distribution Dose Prostate Brachytherapy Disease contained within the prostate gland (T1c - T2a) Small - to - moderate prostate size ( 60 cc) Favorable pelvic anatomy No or limited prior transurethral prostatic resection Minimal obstructive symptoms (I-PSS 15, peak flow 10) V. Definition of Risk Categories -Low Risk -Intermediate Risk -High Risk V. Prostate Cancer Risk Groups Prostate Cancer Risk Groups Clinical tumor stage, Gleason score and PSA used to determine risk groups: (D`Amico) Low risk: Stage T1-2a, Gleason 6, and PSA < 10 ng/mL Intermediate risk: Stage T2b or Gleason 7 or PSA 10-20 ng/mL High risk: Stage > T2c or Gleason 8-10 or PSA > 20 ng/mL VI. Dose-escalation Trials Retrospective Trials – MSKCC IMRT Dose Escalation – Proton Beam Dose Escalation Prospective Randomized Trials – MDACC Randomized Trial (70 Gy vs. 78 Gy) – Harvard Randomized Trial (72 Gy vs. 79.2Gy) Memorial Sloan Kettering Cancer Center IMRT Dose Escalation Began using IMRT in 1996 to facilitate dose escalation high dose XRT using IMRT for localized prostate cancer 561pts. B/w April 1996 & Jan 2000 Median age 68 (range 46-86) Zelefsky MJ, Chan H, et. Al. Journal of Urology Vol. 176, 1415-1419, Oct 2006 Memorial Sloan Kettering Cancer Center IMRT Dose Escalation Escalated eventually to 81 Gy 296 patients (53%) treated w/ short course (3-mo) androgen deprivation therapy to decrease the size of the prostate ADT discontinued at the completion of radiotherapy Zelefsky MJ, Chan H, et. Al. Journal of Urology Vol. 176, 1415-1419, Oct 2006 Memorial Sloan Kettering Cancer Center IMRT Dose Escalation Median f/u: 7 years (range 5 to 9) PSA relapse: – ASTRO definition: 3 consecutive rises after nadir – Houston definition: nadir + 2 None received post-irradiation androgen deprivation or other anti-cancer therapy before documentation of a PSA relapse Zelefsky MJ, Chan H, et. Al. Journal of Urology Vol. 176, 1415-1419, Oct 2006 Memorial Sloan Kettering Cancer Center IMRT Dose Escalation Low Risk T1-2, GS ≤6, PSA ≤10 Zelefsky MJ, Chan H, et. Al. Journal of Urology Vol. 176, 1415-1419, Oct 2006 Memorial Sloan Kettering Cancer Center IMRT Dose Escalation Intermediate Risk T1-2, GS 6, PSA > 10 T1-2, GS >6, PSA 10 T3, GS 6, PSA 10 Zelefsky MJ, Chan H, et. Al. Journal of Urology Vol. 176, 1415-1419, Oct 2006 Memorial Sloan Kettering Cancer Center IMRT Dose Escalation High Risk GS >6, PSA >10 Zelefsky MJ, Chan H, et. Al. Journal of Urology Vol. 176, 1415-1419, Oct 2006 Memorial Sloan Kettering Cancer Center IMRT Dose Escalation Biochemical Control Using the ASTRO definition, the 8-year actuarial PSA relapse-free survival – Favorable risk: 85% – Intermediate risk: 76% – Unfavorable risk: 72% Zelefsky MJ, Chan H, et. Al. Journal of Urology Vol. 176, 1415-1419, Oct 2006 Memorial Sloan Kettering Cancer Center IMRT Dose Escalation Distant metastases – developed in 17 (3%) pts 8-year actuarial likelihood of distant metastases – Favorable 1% – Intermediate 5% – Unfavorable 4%, – (favorable vs. intermediate risk p = 0.03; intermediate vs.. unfavorable risk p = 0.86) Cause specific survival outcomes – Favorable 100% – Intermediate 96% – Unfavorable 84% (p = 0.17) Zelefsky MJ, Chan H, et. Al. Journal of Urology Vol. 176, 1415-1419, Oct 2006 Memorial Sloan Kettering Cancer Center IMRT Dose Escalation Toxicity Rectal: – Grade 2 rectal bleeding: 7 patients (1.5%) – Grade 3 rectal toxicity: 3 patients (<1%) – No grade 4 rectal complications – 8-year actuarial likelihood of late grade > 2 rectal toxicity: 1.6% Urinary: – Late grade 2 chronic urethritis requiring medication for symptom control: 9% – Urethral stricture requiring dilation (gr3): 3% – 8-year actuarial likelihood of late grade > 2 urinary toxicities: 15% Zelefsky MJ, Chan H, et. Al. Journal of Urology Vol. 176, 1415-1419, Oct 2006 Memorial Sloan Kettering Cancer Center IMRT Dose Escalation Toxicity Sexual: – Before the initiation of therapy 403 (72%) patients reported the ability to maintain an erection sufficient for sexual intercourse – In this group of pts ED developed in 49% Secondary Malignancy: – None observed Zelefsky MJ, Chan H, et. Al. Journal of Urology Vol. 176, 1415-1419, Oct 2006 Loma Linda Proton Beam Experience Dose Escalation . B/w Oct 1991 & Dec 1997, 1255 pts with Stages Ia-III prostate cancer No prior surgery, hormonal therapy, or distant mets Treated with protons alone or in combination with photon-beam XRT Slater JD, Rossi CJ, et. Al. IJROBP Vol 59, No. 2, 348-352, 2004. Loma Linda Proton Beam Experience Dose Escalation . Freedom from biochemical evidence of disease (bNED) used ASTRO consensus definition ( 3 consecutive PSA rises after reaching a nadir) Mean duration f/u: 63 months Median age: 69 years Slater JD, Rossi CJ, et. Al. IJROBP Vol 59, No. 2, 348-352, 2004. Loma Linda Proton Beam Experience Dose Escalation Overall 5-year & 8year actuarial biochemical diseasefree survival rates: 75% & 73% Slater JD, Rossi CJ, et. Al. IJROBP Vol 59, No. 2, 348-352, 2004. Comparison of IMRT versus Proton Therapy Median Follow up Pts. 5yr BDFS 8yr BDFS Multivariat e Analysis Late rectal Late Urinary Sexual Dysfunction IMRT 7yrs 561 N/A 85% for LR, 76% for IR, 72% for HR Clinical Stage & PreTreatment PSA significant Gr 2 or greater 1.6%, Gr 3 <1%, no Gr 4 Gr 2 or greater 15%, Gr 3 was 3%, No Gr 4 49% PBRT 62mo 1255 75% 73% PreTreatment PSA, Gleason, & PSA nadir significant Gr 3 <1%, Gr 4 <1% (1pt with Gr 4) Gr 3 or greater <1%, no Gr 4 N/A MDACC Randomized Dose Escalation Trial Results Of A Randomized Dose-Escalation Study Comparing 70 Gy To 78 Gy(isocenter) For The Treatment Of Prostate Cancer Pollack IJROBP 2002 MDACC Randomized Dose Escalation Trial Freedom from Failure by PSA PSA <=10 ng/ml PSA >10 ng/ml 1.0 1.0 78 Gy .9 78 Gy .9 .8 Fraction free of failure .8 .7 .6 .7 .6 70 Gy .5 .4 .3 p = 0.46 .5 .4 .3 .2 .2 .1 .1 0.0 0.0 0 20 40 60 Months after radiotherapy Pollack IJROBP 2002 80 100 p = 0.012 0 20 40 70 Gy 60 Months after radiotherapy 80 100 MDACC Randomized Dose Escalation Trial Fraction Free of Distant Metastases, PSA > 10 1.0 78 Gy .9 70 Gy .8 .7 .6 p = 0.056 .5 0 20 40 60 80 Months after radiotherapy Pollack IJROBP 2002 100 Harvard Randomized Dose Escalation Trial Phase III trial comparing conventional dose with high dose radiation in early stage prostate cancer: results of PROG 95-09 Zietman A, et. al. JAMA, 2005, 294 (10): 1233 Harvard Randomized Dose Escalation Trial Trial design T1b-2b prostate cancer PSA <15ng/ml No hormonal therapy randomization ACR/RTOG Proton boost 19.8 GyE 3-D conformal photons 50.4 Gy Proton boost 28.8GyE 3-D conformal photons 50.4 Gy Total prostate dose Total prostate dose 70.2 GyE 79.2 GyE Harvard Randomized Dose Escalation Trial Freedom from Biochemical Failure (ASTRO definition) 1.0 * Freedom from Biochemical Failure Rate 0.9 79% 0.8 0.7 61% 0.6 * 0.5 0.4 0.3 70.2 GyE 79.2 GyE 0.2 P = <0.0001 0.1 * 95% confidence intervals 0.0 0 1 2 3 4 5 6 7 8 10 20 10 2 Years Since Randomization # at risk 197 195 196 194 171 184 139 163 118 148 76 99 31 46 Harvard Randomized Dose Escalation Trial Freedom from Biochemical Failure (ASTRO definition) Low Intermediate/high 1.0 79% 0.9 79.2GyE 78% 79.2GyE 0.8 0.7 0.6 55% 0.5 61% 70.2GyE 70.2GyE 0.4 0.3 0.2 n = 162 p = 0.03 n = 230 p = <0.001 0.1 0.0 0 1 2 3 4 5 6 7 Years since randomization 8 0 1 2 3 4 5 6 7 Years since randomization Zietman A, et. al. JAMA, 2005, 294 (10): 1233 8 VII. Low Risk Disease Treatment -IMRT alone -Seeds alone VII. Radiotherapy for Low Risk Prostate Cancer Treatment Options for Low- Risk Group Watchful waiting vs. active surveillance Radical prostatectomy IMRT Interstitial brachytherapy Memorial Sloan Kettering Cancer Center IMRT Dose Escalation Low Risk T1-2, GS ≤6, PSA ≤10 Zelefsky MJ, Chan H, et. Al. Journal of Urology Vol. 176, 1415-1419, Oct 2006 Brachytherapy for Low Risk Prostate Cancer Study design 125 pts with T1-T2b treated with I-125 brachytherapy b/w 1988-1990 Gleason < 6 Median PSA 5.1 Endpoint biochemical outcome – Failure is 2 consecutive rises in PSA Grimm P, et. al. IJROBP, 51 (1), 31-40, 2001. Brachytherapy for Low Risk Prostate Cancer Grimm P, et. al. IJROBP, 51 (1), 31-40, 2001. VIII. Intermediate Risk Disease Treatment -IMRT alone -6 mo Hormone + EBRT -Seeds + EBRT VIII. Radiotherapy for Intermediate Risk Prostate Cancer Memorial Sloan Kettering Cancer Center IMRT Dose Escalation Intermediate Risk T1-2, GS 6, PSA > 10 T1-2, GS >6, PSA 10 T3, GS 6, PSA 10 Zelefsky MJ, Chan H, et. Al. Journal of Urology Vol. 176, 1415-1419, Oct 2006 6 mo Hormone + Radiotherapy for Intermediate Risk Prostate Cancer 3DCRT Rx 67Gy normalized to 95% isodose D` Amico A, et al. JAMA. 2004; 292(7): 821-826. 6 mo Hormone + Radiotherapy for Intermediate Risk Prostate Cancer Overall Survival 88% 78% D` Amico A, et al. JAMA. 2004; 292(7): 821-826. IX. High Risk Disease Treatment -Long-term Hormonal therapy Randomized Trials -RTOG randomized Trial -EORTC randomized Trial -Seeds + EBRT IX. Radiotherapy for High Risk Prostate Cancer Memorial Sloan Kettering Cancer Center IMRT Dose Escalation High Risk GS >6, PSA >10 Zelefsky MJ, Chan H, et. Al. Journal of Urology Vol. 176, 1415-1419, Oct 2006 Randomized Trials Involving Hormone Therapy for Locally Advanced Prostate Cancer Trial Eligibility Arms Overall Survival p-value RTOG 8531 T3 (15%) or T1-2, N+ or path T3 and (+) margin or (+) SV RT (HT @ failure) RT + AHT indefinite 10-year 39% v 49% p=.002 EORTC 22863 T3-4 (89%) or T1-2 WHO 3 RT RT+CAHT 3 years 5-year 62% v 78% p=.0002 RTOG 8610 Bulky T2b, T3-4, N+ allowed RT v RT+NHT (TAB) 3.7 mo 8-year 44% v 53% p=.10 RTOG 9202 T2c-4 w/PSA <150, N+ allowed RT+NHT (TAB) 4 mo RT+NHT+AHTx28mo 5-year 70% v 80% GS 8 p=.73 P < .004 GS8 RTOG 9413 T2c-4 w/Gleason>6, or >15% risk of N+ WP+ 4mo NHT WP+ 4mo AHT PO+ 4mo NHT PO+ 4mo AHT 4-year 84.7% v 84.3% p=.94 DFCI T1b-T2b, or mri-T3 PSA=10-40, GS≥7 3DRT 3DRT + 6mo (TAB) 5-year 78% v 88% p=.04 TROG 9601 T2-4, any PSA, any GS RT RT + 3mo NHT Not stated p=ns X. Comparing Modalities X. Can we compare radiation modalities to surgical therapy? No modern clinical trials have successfully compared these modalities – Physicians and patients alike unwilling to accept randomization Different definitions of cancer control Patients are not comparable between modalities, even at same institution Comparison of Outcome by Modality Kupelian, Potters et al, IJROBP 2004 (Cleveland Clinic & MSKCC Mercy Hospital) Comparison of Outcome by Modality Kupelian, Potters et al, IJROBP 2004 (Cleveland Clinic & MSKCC Mercy Hospital) Comparison of Outcome by Modality Multivariate analysis of factors predictive of bRFS Kupelian, Potters et al, IJROBP 2004 (Cleveland Clinic & MSKCC Mercy Hospital) Surgery vs Radiotherapy for Prostate Cancer Risk of Death 7316 men treated at 44 U.S. medical institutions for Stage II prostate cancer Risk of prostate cancer-related death was not measurable affected by type of therapy Intermediate-risk patients with multiple risk factors fared better with RT Relative risk (RR) of death due to prostate cancer D’Amico AV. J Clin Oncol 21:2163-72, 2003 Surgery vs Radiotherapy for Prostate Cancer American Urological Association Prostate Cancer Clinical Guidelines Panel convened to analyze the literature regarding available treatment methods MEDLINE search of 1453 journal articles Analysis confined to 165 “best” articles Articles did not permit valid comparisons of the treatment methods Data from the medical literature do not provide clearcut superiority of any one treatment Middleton RG. J Urol 154:2144-8, 1996. XI. Quality of Life Comparison XI. Quality of Life After Prostate Cancer Therapy Urinary Bothersome 80 Brachytherapy (P < 0.0001) External 60 Surgery Controls % 40 20 0 None Very small Small Moderate Big Wei, JT: J Clin Oncol 20: 557-566, 2002 Quality of Life After Prostate Cancer Therapy Bowel Bothersome 100 % 80 Brachytherapy (P < 0.0001) (P < 0.0001) External 60 Surgery Controls 40 20 0 None Very small Small Moderate Big Wei, JT: J Clin Oncol 20: 557-566, 2002 Quality of Life After Prostate Cancer Therapy Sexual Function Bothersome 60 Brachytherapy (P < 0.0001) 50 40 External (P < 0.0001) Surgery (P < 0.0001) Controls % 30 20 10 0 None Very small Small Moderate Big Wei, JT: J Clin Oncol 20: 557-566, 2002 XII. Conclusions Factors Influencing Management Approach Patient expectations of medical care Patient understanding of & engagement in management of condition Patient preference for type of treatment Access of patient to each member of a multispecialty team Dr. Ko`s Treatment Recommendations Low Risk Pts Surgery or Radiation Intermediate Risk Pts Surgery or Radiation Equivalent in terms of QOL and Outcomes Equivalent in terms of QOL and Outcomes High-Risk Pts Radiation is the treatment of choice in terms of cancer control