A Report from TRANSLATE-ACS - Duke Clinical Research Institute
advertisement
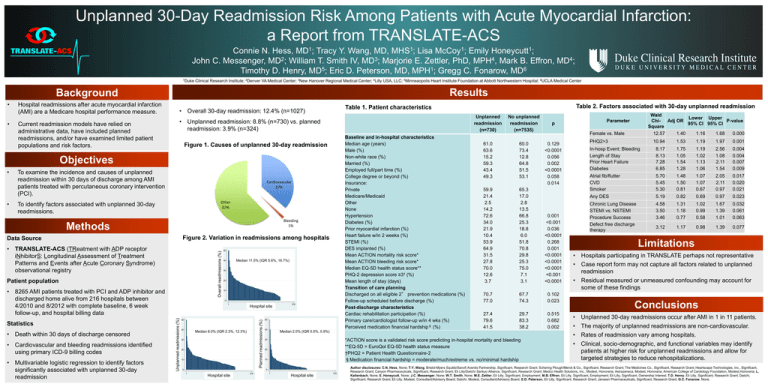
Unplanned 30-Day Readmission Risk Among Patients with Acute Myocardial Infarction: a Report from TRANSLATE-ACS Connie N. Hess, MD1; Tracy Y. Wang, MD, MHS1; Lisa McCoy1; Emily Honeycutt1; John C. Messenger, MD2; William T. Smith IV, MD3; Marjorie E. Zettler, PhD, MPH4, Mark B. Effron, MD4; Timothy D. Henry, MD5; Eric D. Peterson, MD, MPH1; Gregg C. Fonarow, MD6 1Duke Clinical Research Institute; 2Denver VA Medical Center; 3New Hanover Regional Medical Center; 4Lilly USA, LLC; 5Minneaopolis Heart Institute Foundation at Abbott Northwestern Hospital; 6UCLA Medical Center Results Background • • Hospital readmissions after acute myocardial infarction (AMI) are a Medicare hospital performance measure. Current readmission models have relied on administrative data, have included planned readmissions, and/or have examined limited patient populations and risk factors. • Overall 30-day readmission: 12.4% (n=1027) • Unplanned readmission: 8.8% (n=730) vs. planned readmission: 3.9% (n=324) Baseline and in-hospital characteristics Median age (years) Male (%) Non-white race (%) Married (%) Employed full/part time (%) College degree or beyond (%) Insurance: Private Medicare/Medicaid Other None Hypertension Diabetes (%) Prior myocardial infarction (%) Heart failure w/in 2 weeks (%) STEMI (%) DES implanted (%) Mean ACTION mortality risk score* Mean ACTION bleeding risk score* Median EQ-5D health status score** PHQ-2 depression score ≥3† (%) Mean length of stay (days) Transition of care planning Discharged on all eligible 2° prevention medications (%) Follow-up scheduled before discharge (%) Post-discharge characteristics Cardiac rehabilitation participation (%) Primary care/cardiologist follow-up w/in 4 wks (%) Perceived medication financial hardship§ (%) Figure 1. Causes of unplanned 30-day readmission Objectives • • To examine the incidence and causes of unplanned readmission within 30 days of discharge among AMI patients treated with percutaneous coronary intervention (PCI). Cardiovascular 37% Other 62% To identify factors associated with unplanned 30-day readmissions. Bleeding 1% Methods % overall readmissions • TRANSLATE-ACS (TReatment with ADP receptor iNhibitorS: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome) observational registry Patient population • 8265 AMI patients treated with PCI and ADP inhibitor and discharged home alive from 216 hospitals between 4/2010 and 8/2012 with complete baseline, 6 week follow-up, and hospital billing data 30 20 10 1 Hospital site by site rank Hospital 50 40 Planned readmissions (%) • Multivariable logistic regression to identify factors significantly associated with unplanned 30-day readmission Median 11.5% (IQR 5.6%, 16.7%) 40 Median 8.0% (IQR 2.3%, 12.3%) % planned readmissions • Cardiovascular and bleeding readmissions identified using primary ICD-9 billing codes 50 0 Unplanned readmissions (%) • Death within 30 days of discharge censored % unplanned readmissions Statistics Overall readmissions (%) Figure 2. Variation in readmissions among hospitals Data Source 30 20 10 216 50 40 1 Hospital site by site rank Hospital 216 Unplanned readmission (n=730) No unplanned readmission (n=7535) 61.0 63.6 15.2 59.3 43.4 49.3 60.0 73.4 12.8 64.8 51.5 53.1 59.9 21.4 2.5 14.2 72.6 34.0 21.9 10.4 53.9 64.9 31.5 27.8 70.0 12.6 3.7 65.3 17.0 2.8 13.5 66.8 25.3 18.8 6.0 51.8 70.8 29.8 25.3 75.0 7.1 3.1 0.001 <0.001 0.036 <0.0001 0.268 0.001 <0.0001 <0.0001 <0.0001 <0.001 <0.0001 70.7 77.0 67.7 74.3 0.102 0.023 27.4 79.6 41.5 29.7 83.3 38.2 0.515 0.682 0.002 Median 2.0% (IQR 0.0%, 5.9%) *ACTION score is a validated risk score predicting in-hospital mortality and bleeding **EQ-5D = EuroQol EQ-5D health status measure †PHQ2 = Patient Health Questionnaire-2 §Medication financial hardship = moderate/much/extreme vs. no/minimal hardship 30 20 10 0 0 Table 2. Factors associated with 30-day unplanned readmission Table 1. Patient characteristics 1 Hospital site bysite rank Hospital 216 Wald Lower Upper Chi- Adj OR P-value 95% CI 95% CI Square 12.57 1.40 1.16 1.68 0.000 Parameter p Female vs. Male PHQ2>3 0.129 <0.0001 0.056 0.002 <0.0001 0.058 0.014 10.94 1.53 1.19 1.97 0.001 In-hosp Event: Bleeding Length of Stay Prior Heart Failure Diabetes 8.17 8.13 7.28 6.85 1.75 1.05 1.54 1.28 1.19 1.02 1.13 1.06 2.56 1.08 2.11 1.54 0.004 0.004 0.007 0.009 Atrial fib/flutter CVD Smoker 5.70 5.45 5.30 1.48 1.50 0.81 1.07 1.07 0.67 2.05 2.11 0.97 0.017 0.020 0.021 Any DES 5.19 0.82 0.69 0.97 0.023 Chronic Lung Disease STEMI vs. NSTEMI Procedure Success Defect free discharge therapy 4.58 3.50 3.46 1.31 1.18 0.77 1.02 0.99 0.58 1.67 1.39 1.01 0.032 0.061 0.063 3.12 1.17 0.98 1.39 0.077 Limitations • Hospitals participating in TRANSLATE perhaps not representative • Case report form may not capture all factors related to unplanned readmission • Residual measured or unmeasured confounding may account for some of these findings Conclusions • • • • Unplanned 30-day readmissions occur after AMI in 1 in 11 patients. The majority of unplanned readmissions are non-cardiovascular. Rates of readmission vary among hospitals. Clinical, socio-demographic, and functional variables may identify patients at higher risk for unplanned readmissions and allow for targeted strategies to reduce rehospitalizations. Author disclosures: C.N. Hess, None; T.Y. Wang, Bristol-Myers Squibb/Sanofi Aventis Partnership, Significant, Research Grant; Schering Plough/Merck & Co., Significant, Research Grant; The Medicines Co., Significant, Research Grant; Heartscape Technologies, Inc., Significant, Research Grant; Canyon Pharmaceuticals, Significant, Research Grant; Eli Lilly/Daiichi Sankyo Alliance, Significant, Research Grant; Medco Health Solutions, Inc., Modest, Honoraria; Astrazeneca, Modest, Honoraria; American College of Cardiology Foundation, Modest,Honoraria; L. Kaltenbach, None; E. Honeycutt, None; J.C. Messenger, None; W.T. Smith, None; M.E. Zettler, Eli Lilly, Significant, Employment; M.B. Effron, Eli Lilly, Significant, Employment; Eli Lilly, Significant, Ownership Interest; T.D. Henry, Eli Lilly, Significant, Research Grant; Daiichi, Significant, Research Grant; Eli Lilly, Modest, Consultant/Advisory Board; Daiichi, Modest, Consultant/Advisory Board; E.D. Peterson, Eli Lilly, Significant, Research Grant; Janssen Pharmaceuticals, Significant, Research Grant; G.C. Fonarow, None.