ppt
advertisement

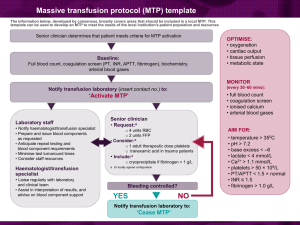
BLOOD COMPONENTS WHAT DO YOU NEED TO KNOW? Janine Carnell Transfusion Nurse Consultant Eastern Health Members of Eastern Health: Angliss Hospital, Box Hill Hospital, Healesville & District Hospital, Maroondah Hospital, Peter James Centre, Turning Point Alcohol & Drug Centre, Wantirna Health, Yarra Ranges Health and Yarra Valley Community Health MYTHS AND LEGENDS Egyptian princes, knights and royalty bathed in it – resuscitate the sick and rejuvenate old and incapacitated Warriors, hunters and Romans drank it – from dying gladiators (such blood was especially beneficial since the athletes were strong and brave) Used as a sacrifice to the Gods by ancient American and Mexican Indians. In England, blood from newly executed criminals was a curative (prescription required). BLOOD TRANSFUSION HISTORY In 1667, Dr. Jean-Baptiste Denys (physician to King Louis XIV of France) transfused the blood of a sheep into a 15y.o. boy who survived the transfusion (small amount) The first successful human-to-human blood transfusion • Many other examples Karl Landsteiner discovered A, B + C (O) groups 1901. • • 1818-1829 by eminent British obstetrician and physiologist James Blundell (PPH: husband to wife). 5 out of 10 of his subsequent transfusions were successful. 4th major group (AB) reported by Decastello and Sturli in 1902. Around 1937, discovery of the Rhesus blood group system – which was found to be the cause of the majority of transfusion reactions up to that time Today, to meet the annual needs of patients, Victoria collects approx: 250,000 blood donations, 78,000 plasma donations and 9,000 platelet donations WHO USES BLOOD? RED CELLS (>200MLS) Treatment of clinically significant anaemia with symptomatic deficit of oxygen carrying capacity Replacement of traumatic or surgical blood loss Paediatric (25-100mls) – infants, young children and intrauterine transfusion (RCH) Special Red cells - i.e. irradiated, washed, fully phenotyped, CMV negative, whole blood, directed donations, autologous blood Use blood of identical ABO and Rh(D) type whenever possible. In an emergency situation, Group O Rh(D) Negative blood should be given while the patient's blood group is being established. One unit of red cells raises the haemoglobin concentration in an average sized adult by approximately 10 g/L. Red cells are filtered to remove most leucocytes and may be resuspended in other additives to prolong storage. Although important, the patient’s haemoglobin level should not be the sole deciding factor for giving red cells. CONSIDER RBC transfusion should not be dictated by a Hb concentration alone, but should also be based on assessment of the patient’s clinical status. Where indicated, transfusion of a single unit of RBC followed by clinical reassessment is appropriate. HAEMOGLOBIN CONSIDERATIONS <70 g/L Lower thresholds may be acceptable in patients without symptoms and/or where specific therapy is available. 70–100 g/L Likely to be appropriate during surgery associated with major blood loss or if there are signs or symptoms of impaired oxygen transport. >80 g/L May be appropriate to control anaemiarelated symptoms in a patient on a chronic transfusion regimen or during marrow suppressive therapy. >100 g/L Not likely to be appropriate unless there are specific indications. PLATELETS Therapeutic – Bleeding due to decreased platelet production or functionally abnormal platelets Prophylaxis – rapidly falling or low platelet counts <10 x 109 /L secondary to cancer or chemotherapy or bone marrow failure without risk factors <20 x 109 /L in bone marrow failure in the presence of additional risk factors (e.g., fever, antibiotic or evidence of systemic haemostatic failure) Maintain >50 x 109 /L for most surgical procedures; and >100 x 109 /L for surgeries with high risk of bleeding (e.g., ocular or neurosurgery) Apheresis (100-400mls) – single donor Pooled (>160mls) – 4 donations Paediatric (40-60mls) – infants, as above One standard dose (1 pack apheresis or 1 pooled) would be expected to increase the platelet count of a 70 kg adult by approx 20–40 x 109 /L NB. ALL platelets from ARCBS in Victoria are universally leucodepleted and irradiated. Check labelling FFP (FRESH FROZEN PLASMA) Patients with coagulopathy who are bleeding when specific therapy (such as Vitamin K or factor concentrates are unavailable) To replace labile plasma coagulation factors during massive transfusion, cardiac bypass, liver disease or acute disseminated intravascular coagulation in the presence of bleeding and abnormal coagulation Double split (250-334mls) Triple Split (250-310mls) Paediatric (~60-80mls) is separated from a single unit of whole blood and then divided into 4 packs. Reduce donor exposure and minimise product wastage Volume depends on clinical situation, patient size and laboratory tests. General guide is 10 -15mL/Kg per dose. Given in addition to Vit.K in warfarin overdose Warfarin Reversal Guidelines (Vit.K, PTX, FFP) CRYOPRECIPITATE Cryo (10-40mls) Treatment of fibrinogen deficiency with clinical bleeding, an invasive procedure, trauma or disseminated intravascular coagulation Massive transfusion Apheresis (60mls) 1 unit of cryoprecipitate apheresis is approximately equivalent to 2 units of cryoprecipitate derived from whole blood A common dose for fibrinogen replacement is 30–40 mL cryoprecipitate for each 10 kg patient body weight (~5 – 10 units) Prepared by thawing FFP and recovering the precipitate. The coldinsoluble precipitate is refrozen. It contains most of the factor VIII, fibrinogen, factor XIII, von Willebrand factor and fibronectin from FFP. BLOOD MANAGEMENT GUIDELINES NHMRC, ASBT, NBA 6 MODULES Module 2: Perioperative Minimise requirement Promote appropriate use Identify high risk patients, and develop a specific management plan ahead of time Clinical Guidance Effect of anaemia on outcomes Effect of blood transfusion on outcomes Cessation of medications that affect haemostasis Peri-operatvie strategies to minimise blood loss Prevent hypothermia, positioning, deliberate hypotension, cell salvage Triggers for transfusion – coagulation parameters Anaesthesia and Patient Blood Management BLOOD MANAGEMENT GUIDELINES Pre-Op management Anaemia – optimise Hb and iron stores Haemostasis management – warfarin, aspirin, NSAIDs considerations Blood Conservation Strategies Pre-op Pre-operative Autologous Donation (PAD) Intra-op Acute Normovolaemic Haemodilution (ANH) Cell Salvage Post-op Cell Salvage Appropriate transfusion practice Transfuse in accordance with clinical practice guidelines INDICATIONS FOR BLOOD IN THEATRE Elective surgery– well planned Emergency – chaotic Acute blood loss Obstetric – blood loss unpredictable (intra-op, post-op) Hb levels and surgery Levels 70-100g/L – transfusion likely to be appropriate if major blood loss or S/S of impaired O2 transport Bleeding disorders (acquired/congenital) – liver disease, aspirininduced platelet dysfunction, DIC, thrombocytopaenia, haemophilia Anti-coagulated patient – be aware MBOS – Maximum Blood Order Schedule – local hospital policy e.g. Arthroscopy (nil), Caesarean (G+S), Open Prostatectomy (2units) RISKS IN THEATRE Checking remains VITAL Emergency or Elective Crossmatched or Emergency Uncrossmatched Unconscious or Unidentified patient Local hospital policy Source - Toonpool.com Remote or Satellite fridge Must have a detailed register of products in and out Audit trail between Blood Bank and Theatre Alarmed, monitored, maintenance schedule Rapid access Protection from public Multiple theatres – multiple units in fridge CURRENT RISKS TRANSFUSION TRANSMITTED INFECTION “Australia has one of the safest blood supplies in the world in terms of viral safety.” – ARCBS Risks of Transfusion-transmitted Infection Calculated on Blood Service Data Agent and testing standard Window period Estimate of residual risk ‘per unit’ HIV (antibody + NAT) 5.6 days Less than 1 in 1 million HCV (antibody + NAT) 3.1 days Less than 1 in 1 million HBV (HBsAg + NAT) 23.9 days Approximately 1 in 764,000 HTLV 1 & 2 (antibody) 51 days Less than 1 in 1 million vCJD [No testing] Malaria (antibody) Possible, not yet reported in Australia 7–14 days Less than 1 in 1 million Notes: vCJD=variant Creutzfeldt-Jakob Disease; (a) The risk estimates for HIV, HCV, HBV and HTLV are based on Blood Service data from 1 January 2010 to 31 December 2011. Source – ARCBS website July 2012 INFORMED CONSENT Informed consent for transfusion means a documented dialogue has occurred between the patient and a prescriber : The reason for the proposed blood product transfusion. The nature of the proposed blood product transfusion – what is involved The risks and benefits of the blood product as well as the risks or consequences of not receiving the product. The availability and appropriateness of any other blood management strategies. An opportunity to ask questions. Use of a competent interpreter when the patient is not fluent in English. Use of written information or diagrams where appropriate. Be aware of local hospital policy Specific consent form? Generic Consent form? Request slip? Progress Notes? Who signs? Doctor? Patient? Witness? Refusal? DOCUMENTATION – BLOOD TRANSFUSION Variation in forms and requirements across Health Services Know hospital policy Request slip Blood release form Specific blood obs, what is documented in notes… Adverse event form / Transfusion Report Form Details of component, patient ID, crossmatch compatibility… Observation charts, Fluid Balance Charts, Progress Notes Date, timing, terminology, special requirements, urgency… Compatibility report form Where, what, who… IV orders/Prescription for blood – specific, detailed Patient details, components required, urgency, modifications, indications, prescribers and collectors signatures… Reactions, errors, also consider Hospital incident reporting systems Consent requirements EQUIPMENT – FILTERS Stored blood contains blood clots and particles that are potentially fatal to the recipient. This can cause pulmonary complications and death It is essential that there is an integral in-line filter in the giving set to administer a blood product This filter (170-200 micron) removes clots and small clumps of debris that may form during collection and storage. All red cells and platelets issued by the Blood Service are leucocyte depleted Therefore additional bedside leucocyte depletion filters are NOT required. Microaggregate filters (pore size 20-40 microns) are intended to remove microscopic debris from stored red blood cells. There is no evidence from controlled trials that they offer clinical benefit and their use is not generally recommended. EQUIPMENT – IV LINES IV Lines Must contain an inline filter (mesh in/above the drip chamber) Primed with normal saline or the blood component Must not be piggy-backed onto another line Attachment to extension tubing on an IV cannula/multi-lumen venous access device is acceptable How many units can be put through a single IV line? Any number provided the flow remains adequate – if the line becomes clogged – change it Usually the entire transfusion episode Must be changed when transfusion is complete/every 12 hours if not yet complete reduce the risk of bacterial growth occurring Generally speaking – if there is mesh in/above the drip chamber – this is an inline blood filter and you DO NOT need any additional filter IV FLUIDS Compatible N/Saline 4% albumin Plasma protein fractions Incompatible x Calcium containing solutions (e.g. Hartmann’s, Haemaccel) – may cause clotting in line x 5% dextrose – may cause red cells to haemolyse x Most medications – need to flush the line before and after administration ABO compatible plasma Morphine,or Ketamine or Pethidine (diluted in normal saline) Gelofusine The only IV fluid universally compatible with blood components is 0.9% sodium chloride (normal saline) EQUIPMENT PUMPS, PRESSURE DEVICES, RAPID INFUSERS, SYRINGE DRIVERS Pumps may be used to prevent problems with slow flow rates or clogging paediatric patients, or those at risk of fluid overload PICC, CVAD monitored hourly throughout the infusion to ensure that expected volume is delivered Pressure Devices and Rapid Infusers used with large volumes of red cells in the setting of critical bleeding usually also warm the red cells Must be monitored at all times during use Syringe Drivers Paediatric use –need to ensure that blood passes through 170-200micron filter Continuous transfusion of coagulation factors EQUIPMENT BLOOD WARMERS Not usually required in routine transfusions The use of a blood warmer is often advised for: o Large volume rapid transfusions of; o o o o o o >50 mL/kg/hour in adults or >15 mL/kg/hour in children Exchange transfusions Plasma exchange for therapeutic apheresis in adults. Intrauterine transfusions, at the discretion of the feto-maternal specialist Patients with clinically significant cold agglutinins Red cells must not be warmed above the set point temperature of the approved device, commonly 41°C. DO NOT warm blood in hot water, microwave, radiators or under your arms! PRE-TRANSFUSION CHECK The last place we can make sure we are giving the right blood to the right patient Consequences of failure in checking process: Right Blood – lucky it arrived for the right patient Wrong blood – compatible – good luck Wrong component – (e.g. CMV negative, irradiated etc.. ) Puts the patient at risk of adverse event – sometimes this can be delayed Wrong blood – incompatible – this can result in severe transfusion reactions and even death PATIENT IDENTIFICATION Local hospital policy No ID Band = No Blood? In emergency situations, a process should be in place for cases where a patient is unable to be identified Hospitals must have a written policy on the requirements for patient identification Many health services will specify: Patient ID – Surname, given name, DOB, UR number Additional identifiers e.g. gender, address, Medicare number Neonates – 2 ID bands? Unconfirmed identity – name changes – “unknown female” 2 approved staff members must carry out the Patient ID check prior to blood transfusion PRE-TRANSFUSION CHECK If ANY discrepancy – DO NOT transfuse All done at the BEDSIDE Blood Bag Patient ID Documentation OBSERVATIONS Temperature, pulse, respiration rate and blood pressure MUST be measured and recorded : Prior to the start of each individual blood component pack administered 15 minutes after commencing administration of each blood component pack When administration of each blood component pack is completed There is no consensus on subsequent frequency of routine vital sign measurement during transfusion, however many institutions stipulate hourly measurements, after the initial 15 minute period, until completion of the transfusion. Regular visual observation throughout the transfusion is essential Blood Pressure, Temperature, Pulse, Respiratory Rate Baseline 15 minutes Hourly from commencement time Stop time Remember – most transfusion reactions occur within the first 15 mins of transfusion TRANSFUSION RATES The infusion rate for blood products depends on the clinical context, age and cardiac status of the patient. In stable, non-bleeding adult patients typical administration durations are: Red cells Platelets FFP Cryoprecipitate 60-180 minutes per unit 15-30 minutes per standard adult dose 30 minutes per unit (i.e. 10-20mL/kg/hr) 30-60 minutes per standard adult dose (i.e. 10-20mL/kg/hr). Note – in an emergency you are limited only by the size of the cannula TIME LIMITS Commence transfusion within 30 minutes of arrival from Blood Bank If returned to the BB within 30mins it can be returned to the fridge and then released to any other patient as required. Once it has been at room temp for >30mins it cannot then be used for any other patient – you then have up to 4 hrs MAXIMUM to complete the transfusion Transfusion must be complete within 4 hours Risk of bacterial proliferation greatly increases when unit is at room temp for >4 hours TRANSFUSION REACTIONS Local hospital policy Recognise, React and Report Most common adverse reaction = fever Mild Reactions include: Moderate to Severe reactions include: Fever, rash, Fever, hypotension/shock OR hypertension, tachycardia, tachypnoea, wheeze, stridor, rigors or chills, nausea, vomiting or pain (local, chest, back) Reporting requirements Flow chart to follow? Report form to complete? Investigations required? Documentation? TRANSFUSION REACTION IN AN UNCONSCIOUS PATIENT May occur after only 5-10 ml In a rapid emergency transfusion – signs may only be evident after several units Extra care must be taken in the unconscious patient to monitor and react to changes in vital signs Possible Signs of a Severe Adverse Reaction to Transfusion in an Unconscious Patient Temperature rise more than 10C Pink or red urine (hemoglobinuria) Increased operative bleeding Hypotension Reduced urine output Tachycardia or bradycardia NURSING MANAGEMENT Recognise React that signs and symptoms may be due to the transfusion immediately STOP transfusion assess and manage patient, follow policy review documentation (in particular check the pt’s ID band against blood bag and compatibility report form) Report to HMO and Blood Bank Document: (local hospital policy) Transfusion Report Form and/or Incident report Progress notes Investigations – pathology slip TRANSFUSION REACTION FLOW CHART MANY OTHER RESOURCES ARE AVAILABLE RESOURCES / ACRONYMS Australian Red Cross Blood Service (ARCBS) Australian and New Zealand Society of Blood Transfusion (ANZSBT) http://www.transfusion.com.au/ http://www.anzsbt.org.au/ Australasian Society of Blood Transfusion (ASBT) National Blood Authority (NBA) http://www.nba.gov.au/ National Health and Medical Research Council (NHMRC) http://www.nhmrc.gov.au/guidelines/publications/cp78 Blood Matters Program – Department of Health http://www.health.vic.gov.au/bloodmatters/ Local hospital policies QUESTIONS? Janine Carnell Transfusion Nurse Consultant Eastern Health Members of Eastern Health: Angliss Hospital, Box Hill Hospital, Healesville & District Hospital, Maroondah Hospital, Peter James Centre, Turning Point Alcohol & Drug Centre, Wantirna Health, Yarra Ranges Health and Yarra Valley Community Health