Management of Stage III NSCLC - Arizona Center for Cancer Care
advertisement

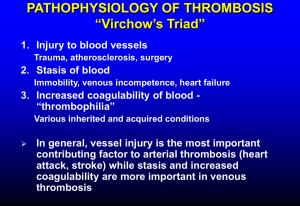
James Choi, MD Hematology/Oncology Arizona Center for Hematology/Oncology Glendale, Arizona Financial Disclosures Speaker for Bayer Healthcare. Learning Objectives Overview of thrombophilia. Indication for initiating a workup. Risk stratification of thrombophilia. Duration of anticoagulation. Venous Thromboembolism Most common presentation is DVT of the lower extremity and PE A risk factor for thromboembolism is found in 80% of patients Often more than one factor at play Divided into hereditary and acquired Risk Factors of Venous Thrombosis Acquired Inherited Mixed/Unknown Advanced Age Antithrombin Deficiency Homocysteine Immobilization Protein C Deficiency Factor 8 Major Surgery Protein S Deficiency Malignancy Factor 5 Leiden mutation Factor 9 Estrogen/Pregnancy (OCP, HRT, SERMs) Prothrombin G20210A mutation Factor 11 APC resistance in absence of FVL Antiphospholipid antibody syndrome TAF1 MPD, PNH Free TPI IBD, Nephrotic syndrome,AAV Fibrinolytic Activity Heparin Induced Thrombocytopenia Obesity Prolonged Air Travel TPI: Tissue pathway inhibitor Prevalence of Major Hypercoagulable States Inherited Thrombophilias Inherited hypercoaguable states A genetic tendency for venous thromboembolism Should be suspected in anyone who: ◦ Presents with an unprovoked venous or arterial thromboembolic disease at <45 yrs ◦ 2 or more thrombotic episodes in the absence of a risk factor for thrombosis ◦ History of objectively confirmed idiopathic thrombosis in first-degree relative ◦ Thrombosis in an unusual site Mesenteric veins, dural sinus ◦ Neonatal thromobosis or stroke ◦ History of recurrent fetal loss Inherited Thrombophilias In the recent past, a genetic cause for thrombophilia was detected in only 5-15% of patients Limited to antithrombin gene deficiency, protein C+S deficiency, dysfibrogenemia 1993: Discovered Factor V gene mutation (Factor V Leiden) 1996: Discovered the prothrombin G20210A gene mutation Inherited thrombophilias are most common in Caucasians, rare in African or Asian In all comers with DVT, the incidence of inherited thrombophilias is 24-37% Of Caucasian patients who present with initial symptomatic DVT, 1220% are heterozygous for factor V Leiden and 6% heterozygous for prothrombin G20210A mutation Inherited thrombophilias More than 50% of cases, thrombosis is provoked by surgery, pregnancy, immobilization, OCP, HRT, or old age Recurrent venous thrombosis is highest in deficiency of antithrombin, protein C, protein S, greater than one inherited thrombophilia, homozygous for factor V Leiden Major Mechanisms Involved in the Normal Control of Coagulation and Inherited Thrombophilias In inherited thrombophilias, thrombosis is most often caused by impaired neutralization of thrombin or failure to control the generation of thrombin Seligsohn U and Lubetsky A. N Engl J Med 2001;344:1222-1231 Factor V Leiden Factor V is activated to Va, which acts as a cofactor in the conversion of prothrombin to thrombin Normally, Factor Va is degraded by APC and limits prothrombin conversion to thrombin Arginine is replaced by Glutamine (Arg506Gln) on the factor V gene, resulting in a protein called factor V Leiden Factor V Leiden is less susceptible to inactivation by APC and is now considered “resistant to APC” This results in a prothrombotic state Factor V Leiden Most common - 40-50% of inherited thrombophilias Found in 5% of the Caucasian population Found in 10-20% of patients with first episode of idiopathic DVT Found in 50% of patients with recurrent DVT 90-95% of those with factor V Leiden are heterozygous Homozygotes have a more severe course Acquired forms of APC resistance found in pregnancy, use of OCPs, elevated Factor VIII or those with antiphospholipid antibodies Factor V Leiden Anticoagulation therapy Long term therapy not recommended in heterozygotes At no higher risk of recurrent thrombosis than those without the mutation In homozygotes, should use prophylaxis in high risk settings Heterozygous, pregnant women with no history of thrombosis are at low risk for thrombosis Anticoagulation is not recommended Recommendations differ if have a history of thrombosis or homozygous Prothrombin G20210A Mutation A Vitamin K-dependant protein synthesized in the liver Due to substitution of adenine for guanine Results in 30% higher prothrombin levels This promotes generation of thrombin and impairs inactivation of Factor Va by APC Found in 2% of the Caucasian population Seen in 6-10% of patients presenting with first episode of unprovoked DVT Like with factor V Leiden, there is no increased risk of recurrent DVT in heterozygotes Protein C deficiency Seligsohn U and Lubetsky A. N Engl J Med 2001;344:1222-1231 Protein C Deficiency Vitamin K dependent glycoprotein produced in the liver Thrombin binds to thrombomodulin, a protein on the endothelial cell surface This complex converts protein C to activated protein C (APC) which degrades Factors Va and VIIIa, limiting thrombin production Thrombosis occurs when levels drop < 50% Can be seen after surgery, trauma, pregnancy, OCP, liver/renal failure, DIC, or warfarin use Seligsohn U and Lubetsky A. N Engl J Med 2001;344:1222-1231 Protein S Deficiency Vitamin K-dependant protein Circulates as both a free protein (40%) and bound C4b- binding protein (60%), which is part of the classic complement system Only free Protein S can act as a cofactor to APC for the inhibition of Factors Va and VIIIa C4b is increased in acute phase reactions, causing free Protein S to be decreased Like in Protein C deficiency, homozygous patients present soon after birth with neonatal purpura fulminans Protein S Deficiency Decreased levels of Protein S in Liver disease Renal disease Women – especially those on OCPs or pregnant IBD Neonates, infants 50% of heterozygotes experience DVT by 35yrs May have atypical presentation: migraine headache, mesenteric vein thrombosis Antithrombin III Deficiency A vitamin K-independent protein that works inhibit thrombin Prevalence: 1:2000-1:5000 persons 30% of heterozygotes develop a thrombosis by 30yrs, 65% by age 50yrs Homozygous deficiency is almost always incompatible with life 60% will have recurrent thrombosis Risk of thrombosis is particularly high in pregnancy Heparin prophylaxis recommended throughout pregnancy and coumadin for 6 weeks postpartum Antiphospholipid Syndrome Defined by the occurrence of at least one clinical feature AND the presence of at least one type of autoantibody known as an aPL Clinical Criteria: - Arterial or venous thrombosis. - Pregnancy morbidity. Laboratory Criteria: confirmed on 2 or more occasions at least 12 weeks apart. - IgG or IgM anticardiolipin antibody (med-high) - Lupus anticoagulant - Anti-Beta2 Glycoprotein antibodies Plasma Homocysteine Measurement of fasting total plasma homocysteine is no longer recommended There is no data supporting a change in the duration or type of therapy for a patient with hyperhomocysteinemia and a past history of a VTE since homocysteine may be a marker for VTE rather than a cause Results from the Leiden MEGA study indicate that the presence of MTHFR mutation is not associated with an increased risk for VTE Testing for hypercoaguable states Acquired and genetic causes frequently overlap Who should be tested ? What tests should be ordered? When should they be ordered? Who should be tested? Idiopathic (i.e., spontaneous) VTE VTE at young age (<45 years) Recurrent VTE VTE in unusual sites VTE in the setting of a strong family history of VTE Recurrent pregnancy loss (> 3 consecutive 1st trimester pregnancy losses without an intercurrent term pregnancy) Testing should be strongly considered for patients who present with two or more of these criteria. It may also be considered for select asymptomatic individuals, particularly female relatives of patients with known inherited hypercoagulability, provided that the results will affect their decision to begin oral contraceptive pill (OCP) use or hormone replacement therapy (HRT). Hypercoaguable workup APC resistance screen Clotting assay, then confirm with a genetic test Prothrombin G20210A mutation Genetic test (PCR) Functional assay for Protein C + S, antithrombin III deficiency Heterozygous deficiencies are from many different mutations and abnormalities Measure both free and total Protein S Affected by acute thrombosis and anticoagulation, so check levels at least 2 weeks after completing therapy Anticardiolipin and lupus anticoagulant clotting assay Testing should be done at least 2 weeks after completion of anticoagulation Hypercoaguable workup In the setting of acute clot or therapy: Coumadin reduces protein C and S levels Heparin can reduce antithrombin levels Heparin and coumadin make testing for lupus anticoagulant and APC unreliable Sepsis is associated with reduction in levels of protein C, protein S, antithrombin DVT/PE Without evidence of underlying causative factors such as recent surgery, trauma or known malignancy. 1. 2. 3. Obtain complete medical history. Perform physical exam. CBC, CMP. Yes Do findings suggest occult malignancy? Test for the most common coagulation disorders: 1.Factor V Leiden mutation. 2.Prothrombin gene mutation. 3.Antiphospholipid antibody syndrome. Do test results confirm existence of one of these disorders? Consider testing for less common coagulation disorders. 1.Protein C deficiency. 2.Protein S deficiency. 3.Antithrombin III deficiency. Further workup as indicated No Does any of the following apply? 1.Less than 45 years old. 2.Family history of VTE. 3.Recurrent VTE. 4.Idiopathic or unusual site. Factor V Leiden Homozygous : longer therapy Prothrombin mutation Antiphospholipid syndrome Consider longer therapy with higher INR. Management of thrombophilia Risk Classification Management High Risk Indefinite anticoagulation 2 or more spontaneous events 1 spontaneous life-threating event (near fatal PE, cerebral, mesenteric, portal vein thombosis) 1 spontaneous event in association with antiphospholipid antibody syndrome, antithrombin III deficiency, or more than 1 genetic defect Moderate Risk 1 event with a known provocative stimulus Asymptomatic Vigorous prophylaxis in high risk setting Recommendations Duration of Therapy Patient Characteristics Risk of Recurrence Duration of Therapy Major transient risk factor 3% 3 months Minor risk factor; no thrombophilia < 10% if risk factor avoided 6 months until factor resolves Idiopathic, no or low risk thrombophilia < 10% 6 months Idiopathic, high risk thrombophilia > 10% Indefinite More than one idiopathic event > 10% Indefinite Cancer, other ongoing risk factor > 10% Indefinite. Consider LMWH for malignancy References 1. Lijfering. WM et al. Br J Haematol 2010; 149:824. 2. FR Rosendaal - 2005 ASH educational handbook. 3. Goldhaber SZ et al. J Am Coll Cardiol 2010;56:1. 4. Kitchens CS et al. Semin Thromb Hemost. 1985, 11: 293- 315. 5. Seligsohn U et al. N Engl J Med. 2001, 344: 1222-1231. 6. Dickson BC et al. Univ Toronto Med J 2004; 81:166. 7. Bertina RM. Genetic approach to thrombophilia. Thromb Haemost 2001; 86:92.