Document
advertisement

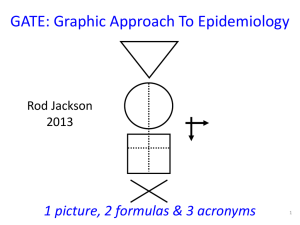
Medical evidence increasing at epidemic rates: we all need EBP skills to keep up-to-date Bastian, Glasziou, Chalmers (2010) 75 Trials and 11Systematic Reviews a Day: How Will We Ever Keep Up? PLoS Med 7(9) Medical evidence increasing at epidemic rates: we all need EBP skills to keep up-to-date approx 75 new trials published every day Bastian, Glasziou, Chalmers (2010) 75 Trials and 11Systematic Reviews a Day: How Will We Ever Keep Up? PLoS Med 7(9) Medical evidence increasing at epidemic rates: we all need EBP skills to keep up-to-date MEDLINE 2010 2,000 articles / day approx 75 new trials published every day Bastian, Glasziou, Chalmers (2010) 75 Trials and 11Systematic Reviews a Day: How Will We Ever Keep Up? PLoS Med 7(9) About 10% of published evidence is worth reading About 1/3 of worthwhile evidence is eventually refuted or attenuated About 1/2 of relevant evidence is not implemented Rapid critical appraisal using GATE Rod Jackson University of Auckland, NZ August 2011 6 Graphic Appraisal Tool for Epidemiological studies Graphic Approach To Evidence Based Practice Graphic Approach To Epidemiology the GATE frame the shape of every epidemiological study 8 8 British doctors smoking status measured smokers Lung cancer non-smokers yes no 5 years Longitudinal (cohort) study 9 British doctors smoking status measured smokers Lung function non-smokers normal abnormal Cross-sectional study 10 British doctors Randomised to aspirin or placebo aspirin placebo yes Myocardial infarction no 5 years RCT 11 Middle-aged US women Test applied Mammogram positive Mammogram negative yes Breast cancer no Clinical use of a diagnostic test 12 Middle-aged US women Breast cancer no Breast cancer positive mammogram negative Diagnostic test accuracy study 13 GATE: Graphic Appraisal Tool for Epidemiological studies 1 picture, 2 formulas & 3 acronyms 14 14 One picture: the GATE frame every epidemiological study hangs on the GATE frame 15 The 1st acronym = PECOT : the 5 parts of every epidemiological study P Participants Exposure Group E C O Time Comparison Group Outcomes T 16 Lewis RT et al. Should antibiotic prophylaxis be used routinely in clean surgical procedures: A tentative yes. Surgery 1995;118:742-7. 17 Background. The incidence of surgical site infection (SSI) after clean surgical procedure is regarded as too low for routine antibiotic prophylaxis. But risk of SSI can be as high as 20%. We assessed the value of prophylactic cefotaxime in patients stratified for risk of SSI in a double-blind RCT. Methods. Patients having clean elective operations were stratified for risk & randomized to receive IV cefotaxime 2 gm or placebo before operation & followed for 4-6 weeks for SSI. Results. The 378 of 775 patients who received cefotaxime had 70% fewer SSIs than those who did not --Mantel-Haenszel risk ratio (MH-RR) 0.31; 95 % CI 0.11 to 0.83. Benefit was clear in the 616 low risk patients--0.97% versus 3.9% SSI (MH-RR 0.25, CI 0.07 to 0.87, p = 0.018), but only a trend was seen in 136 high risk patients--2.8% versus 6.1% SSI (MH-RR 0.48, CI 0.09 to 2.5). Conclusions. The results indicate clear benefit for routine antibiotic prophylaxis in clean surgical procedures. High risk 18 patients need more study. 19 1st critical appraisal task: describe study’s design by hanging on GATE frame using PECOT acronym P E C O T 20 Participants Study Setting Eligible Participants P Participants 21 Lewis Trial Participants Study Setting: patients admitted to QE Hospital, Montreal, Canada (1992-5?) Eligible Participants: undergoing clean surgery or simple cholecystectomy P Participants: 633 (?consecutive eligible patients) 22 Exposure & Comparison Groups Exposure or Intervention Group (EG) EG CG Comparison or Control Group (CG) 23 Exposure & Comparison Groups: low risk group 633 316 Exposure or Intervention Group (EG): 2g cefotaxime IV preop 317 Comparison or Control Group (CG): Identical placebo IV 24 Exposure & Comparison Groups: low risk group 633 316 317 Exposure or Comparison or Intervention Group Control Group (EG): (CG): 308* 308* 2mg cefotaxime IV Identical placebo pre-op IV * With complete follow-up 25 Outcomes (O) yes a b O ‘Dis-ease’ no c Outcomes (O) d 26 633 Outcomes (O) 316 Surgical site infection (SSI) yes 317 a= 3 b= 12 O no c d Primary Outcome (O) 27 Time (T) incidence T prevalence 28 Time (T) 316 317 Outcome: SSI incidence T= time from initiation of treatment to end of follow-up 29 Study design: GATE frame & PECOT Participants P Exposure Group E C O Time T Comparison Group Outcomes 30 Lewis Setting: QE Hospital, Montreal Eligible: clean surgery or cholecystectomy Participants 633 316 Exposure Group: IV cefotaxime Time: Up to 6 wks post-op 317 308 308 3 12 Comparison Group: IV placebo Outcomes: SSI 31 Questions? 32 The 1st formula: study analyses Occurrence (risk) of disease = Numerator ÷ Denominator D N 33 All epidemiological studies involve measuring the OCCURRENCE of ‘outcomes’ D Denominator (Participants) N Numerator (Outcomes) Occ = N÷D 34 All epidemiological studies involve measuring the OCCURRENCE of ‘outcomes’ D Denominator (Participants) T During what period of time (T) was N measured? (incidence) N Numerator (Outcomes) Occ = N÷D (T?) 35 All epidemiological studies involve measuring the OCCURRENCE of ‘outcomes’ Denominator (Participants) D T At what point in time (T) was N measured? (prevalence) N Numerator (Outcomes) Occ = N÷D (T?) 36 The 1st formula: Occurrence (risk) = Numerator ÷ Denominator P Exposed Group Comparison Group DE DC T NE NC O T 37 2nd appraisal task: describe analyses by hanging numbers on the GATE frame and calculating occurrences in exposure & comparison groups P Denominator 1: Exposure Group (EG) Numerator 1: a EG CG a c O b Denominator 2: Comparison Group (CG) Numerator 2: b d 38 Occurrence = N ÷ D P Denominator 1: Exposure Group EG Numerator 1: a EG CG a c Exposure Group Occurrence: EGO = a ÷ EG O b d Denominator 2: Comparison Group CG Numerator 2: b Comparison Group Occurrence: CGO = b ÷ CG 39 Occurrence = N ÷ D P Denominator 1: Exposure Group EG Numerator 1: a EG CG a c Exposure Group Occurrence: EGO = a ÷ EG O b d Denominator 2: Comparison Group CG Numerator 2: b Comparison Group Occurrence: CGO = b ÷ CG 40 Occurrence = N ÷ D P Denominator 1: Exposure Group EG Numerator 1: a EG CG a c Exposure Group Occurrence: EGO = a ÷ EG O b d Denominator 2: Comparison Group CG Numerator 2: b Comparison Group Occurrence: CGO = b ÷ CG 41 Calculate EGO & CGO for SSI in low risk group P Denominator 1: Exposure Group EG = 316 Numerator 1: a=3 EG CG a c O b d Denominator 2: Comparison Group CG = 317 Numerator 2: b = 12 EGO = 3/316= 9.5/1000 at 6 CGO = 12/317 = 37.9/1000 weeks at 6 weeks 42 ITT (intention to treat) analysis Calculate EGO & CGO for SSI in low risk group P Denominator 1: Exposure Group EG = 308 Numerator 1: a=3 EG CG a c O b d Denominator 2: Comparison Group CG = 308 Numerator 2: b = 12 EGO = 3/308= 9.7/1000 at 6 CGO = 12/308 =39/1000 at weeks 6 weeks 43 OT (on treatment) or per-protocol analysis Describing differences between occurrences Relative difference or Relative Risk = EGO ÷ CGO Absolute Difference or Risk Difference = EGO - CGO Number Needed To Treat (NNT) = 1 ÷ RD 44 Describing differences between occurrences (SSI in low risk patients) Relative difference or Relative Risk = EGO ÷ CGO = 9.5/1000 ÷ 37.9/1000 = 0.25 Absolute Difference or Risk Difference = EGO - CGO = 9.5/1000 - 37.9/1000 = -28.4/1000 Number Needed To Treat (NNT) = 1 ÷ RD = 1 ÷ (- 28.4 /1000) = - 1000/28.4 = 35 ‘if 35 patients were given IV cefotaxime pre-op, there would be 1 fewer SSI up to 6 weeks post-op’ 45 Study analyses it’s all about EGO & CGO 46 Questions? 47 The 2nd acronym = RAMBO* : assessing bias ‘strength of study’ P P Recruitment Allocation E E C C Maintenance O Blind or O T T Objective outcomes measurement 48 48 * Paul Glasziou The 2nd acronym = RAMBO* : assessing non random error (i.e. bias) P P Recruitment Allocation E E C C Maintenance O Blind or O T T Objective outcomes measurement 49 * Paul Glasziou 49 3rd appraisal task: assess the degree of bias by applying the RAMBO acronym P P Recruitment Allocation E E C C Maintenance O Blind or O T T Objective outcomes measurement 50 Study setting RAMBO Eligible people P P E C O were Recruitment processes appropriate to study goals? • Study setting & eligibility criteria well described? e.g. Recruit random/representative sample OR consecutive eligibles OR volunteers from advertisements • Participants representative of eligibles? • Prognostic/risk profile appropriate to study question? T 51 RAMBO: A is for Allocation Was Allocation to EG & CG successful? RCT: Allocate randomly by Cohort: Allocate by investigators (e.g drugs) measurement (e.g. smoking) EG CG were EG & CG similar at baseline? O T EG CG O T 52 P EG CG O RAMBO were Participants Maintained as allocated? did most participants remain in allocated groups (EG & CG) Participants &/or investigators blind to exposure (and comparison exposure)? Compliance high & similar in EG & CG? Contamination low & similar in EG & CG? Co-interventions low & similar in EG & CG? T Completeness of follow-up high & similar in EG & CG? 53 P RAMBO Were outcomes measured Blind or Objectively? EG CG O T If outcome measurements not Objective (eg. automated or definitive) were investigators Blind to exposure (and comparison exposure) 54 The 4 (GATE) study biases P Recruitment bias Allocation bias E T C O Maintenance bias Outcomes Measurement bias 55 Questions? 56 The 2nd formula: assessing random error Random error = 95% Confidence Interval(1.96 x Standard Error) 57 4th appraisal task: assess degree of random error in study findings using the 2nd formula Random error = 95% Confidence Interval For the Outcome SSI (low risk group) : EGO = 9.5/1000; (95% CI = 3.2 to 27.5) CGO = 37.9/1000; (95% CI = 21.8 to 65) EGO÷CGO = 0.25 (0.07 to 0.88) EGO-CGO = -28.4 (-52 to -4.8) NNT = -35 (-19 to -211) 58 Excel CATs & paper Gate-lites There is a GATE for every study design www.epiq.co.nz 59 59 Final appraisal task: search for & appraise SRs / meta-analyses using 3rd acronym (FAITH) • Find appropriate studies? • Appraise selected studies? • Include only valid studies? • Total-up (synthesise) appropriately? • Heterogeneity adequately addressed? 60 Systematic Reviews There are 4 Cochrane SRs on this topic and the findings are not consistent 61 Using GATE as a framework for evidence based practice The first 4 steps of EBP 1. Ask a focused question. 2. Access (systematically search for) epidemiological evidence to help answer question. 3. Appraise evidence found for its validity, effect size, precision (ideally all the relevant evidence) 4. Apply the evidence: a. amalgamate the valid evidence with other relevant information (patient/community values, clinical/health issues, & policy context) and make an evidence-based decision; and b. act (implement) the decision in practice EBP Step 1: Ask- turn your question into a 5-part PECOT question Participants (the patient problem) Exposure (e.g. a therapy) Comparison (there is always an alternative! another therapy or no treatment… Outcome (e.g. a disease you want to prevent or manage) Time frame (over which you expect a result) EBP Step 2: Access the evidence – use PECOT to choose search terms Participants (the patient problem) Exposure (e.g. a therapy) Comparison (there is always an alternative! another therapy or no treatment… Outcome (e.g. a disease you want to prevent or manage) Time frame (over which you expect a result) 65 EBP Step 3: Appraise the evidence ‘using the best evidence from epidemiology to help inform decisions’ more critically (using GATE) more systematically (using FAITH) EBP Step 4: APPLY the evidence by: a. AMALGAMATING the relevant information & making an evidence-based decision:’ the X-factor © X-factor: making evidence-based decisions Evidence Clinical / health considerations Patient / community preferences Policy issues Xpertise: ‘putting it all together’ the art of practice