The Radiological Physics Center`s Anthropomorphic Quality
advertisement
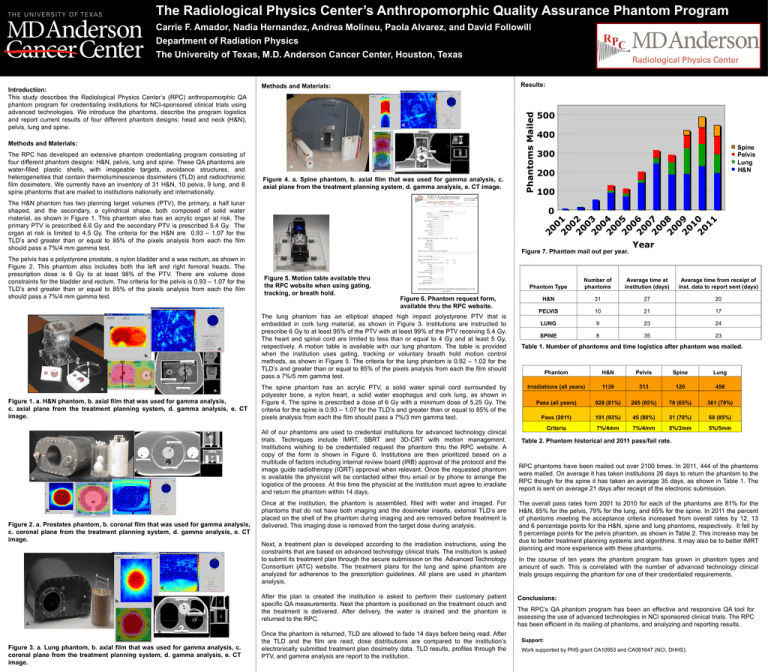
The Radiological Physics Center’s Anthropomorphic Quality Assurance Phantom Program Carrie F. Amador, Nadia Hernandez, Andrea Molineu, Paola Alvarez, and David Followill Department of Radiation Physics The University of Texas, M.D. Anderson Cancer Center, Houston, Texas d. b. Esophagus Anterior Right Left Methods and Materials: The RPC has developed an extensive phantom credentialing program consisting of four different phantom designs: H&N, pelvis, lung and spine. These QA phantoms are water-filled plastic shells, with imageable targets, avoidance structures, and heterogeneities that contain thermoluminescence dosimeters (TLD) and radiochromic film dosimeters. We currently have an inventory of 31 H&N, 10 pelvis, 9 lung, and 8 spine phantoms that are mailed to institutions nationally and internationally. Spine Bone a. c. Posterior Cord e. Figure 4. a. Spine phantom, b. axial film that was used for gamma analysis, c. axial plane from the treatment planning system, d. gamma analysis, e. CT image. The H&N phantom has two planning target volumes (PTV), the primary, a half lunar shaped, and the secondary, a cylindrical shape, both composed of solid water material, as shown in Figure 1. This phantom also has an acrylic organ at risk. The primary PTV is prescribed 6.6 Gy and the secondary PTV is prescribed 5.4 Gy. The organ at risk is limited to 4.5 Gy. The criteria for the H&N are 0.93 – 1.07 for the TLD’s and greater than or equal to 85% of the pixels analysis from each the film should pass a 7%/4 mm gamma test. The pelvis has a polystyrene prostate, a nylon bladder and a wax rectum, as shown in Figure 2. This phantom also includes both the left and right femoral heads. The prescription dose is 6 Gy to at least 98% of the PTV. There are volume dose constraints for the bladder and rectum. The criteria for the pelvis is 0.93 – 1.07 for the TLD’s and greater than or equal to 85% of the pixels analysis from each the film should pass a 7%/4 mm gamma test. d. b. Anterior Primary PTV Right Left Phantoms Mailed Introduction: This study describes the Radiological Physics Center’s (RPC) anthropomorphic QA phantom program for credentialing institutions for NCI-sponsored clinical trials using advanced technologies. We introduce the phantoms, describe the program logistics and report current results of four different phantom designs: head and neck (H&N), pelvis, lung and spine. Results: Methods and Materials: 500 400 Spine Pelvis Lung H&N 300 200 100 0 Figure 7. Phantom mail out per year. Figure 5. Motion table available thru the RPC website when using gating, tracking, or breath hold. Figure 6. Phantom request form, available thru the RPC website. The lung phantom has an elliptical shaped high impact polystyrene PTV that is embedded in cork lung material, as shown in Figure 3. Institutions are instructed to prescribe 6 Gy to at least 95% of the PTV with at least 99% of the PTV receiving 5.4 Gy. The heart and spinal cord are limited to less than or equal to 4 Gy and at least 5 Gy, respectively. A motion table is available with our lung phantom. The table is provided when the institution uses gating, tracking or voluntary breath hold motion control methods, as shown in Figure 5. The criteria for the lung phantom is 0.92 – 1.02 for the TLD’s and greater than or equal to 85% of the pixels analysis from each the film should pass a 7%/5 mm gamma test. Year Phantom Type Number of phantoms Average time at institution (days) Average time from receipt of inst. data to report sent (days) H&N 31 27 20 PELVIS 10 21 17 LUNG 9 23 24 SPINE 8 35 23 Table 1. Number of phantoms and time logistics after phantom was mailed. Phantom H&N Pelvis Spine Lung Irradiations (all years) 1139 313 120 458 Pass (all years) 928 (81%) 265 (85%) 78 (65%) 361 (79%) Pass (2011) 101 (93%) 45 (80%) 31 (78%) 68 (85%) Criteria 7%/4mm 7%/4mm 5%/3mm 5%/5mm Secondary PTV c. Posterior a. Organ at risk e. Figure 1. a. H&N phantom, b. axial film that was used for gamma analysis, c. axial plane from the treatment planning system, d. gamma analysis, e. CT image. Primary PTV Femoral Head Femoral Head Rectum e. a. Rectum Superior Left Right b. c. Inferior d. Figure 2. a. Prostates phantom, b. coronal film that was used for gamma analysis, c. coronal plane from the treatment planning system, d. gamma analysis, e. CT image. The spine phantom has an acrylic PTV, a solid water spinal cord surrounded by polyester bone, a nylon heart, a solid water esophagus and cork lung, as shown in Figure 4. The spine is prescribed a dose of 6 Gy with a minimum dose of 5.25 Gy. The criteria for the spine is 0.93 – 1.07 for the TLD’s and greater than or equal to 85% of the pixels analysis from each the film should pass a 7%/3 mm gamma test. All of our phantoms are used to credential institutions for advanced technology clinical trials. Techniques include IMRT, SBRT and 3D-CRT with motion management. Institutions wishing to be credentialed request the phantom thru the RPC website. A copy of the form is shown in Figure 6. Institutions are then prioritized based on a multitude of factors including internal review board (IRB) approval of the protocol and the image guide radiotherapy (IGRT) approval when relevant. Once the requested phantom is available the physicist will be contacted either thru email or by phone to arrange the logistics of the process. At this time the physicist at the institution must agree to irradiate and return the phantom within 14 days. Once at the institution, the phantom is assembled, filled with water and imaged. For phantoms that do not have both imaging and the dosimeter inserts, external TLD’s are placed on the shell of the phantom during imaging and are removed before treatment is delivered. This imaging dose is removed from the target dose during analysis. Next, a treatment plan is developed according to the irradiation instructions, using the constraints that are based on advanced technology clinical trials. The institution is asked to submit its treatment plan through the secure submission on the Advanced Technology Consortium (ATC) website. The treatment plans for the lung and spine phantom are analyzed for adherence to the prescription guidelines. All plans are used in phantom analysis. Table 2. Phantom historical and 2011 pass/fail rate. RPC phantoms have been mailed out over 2100 times. In 2011, 444 of the phantoms were mailed. On average it has taken institutions 26 days to return the phantom to the RPC though for the spine it has taken an average 35 days, as shown in Table 1. The report is sent on average 21 days after receipt of the electronic submission. The overall pass rates form 2001 to 2010 for each of the phantoms are 81% for the H&N, 85% for the pelvis, 79% for the lung, and 65% for the spine. In 2011 the percent of phantoms meeting the acceptance criteria increased from overall rates by 12, 13 and 6 percentage points for the H&N, spine and lung phantoms, respectively. It fell by 5 percentage points for the pelvis phantom, as shown in Table 2. This increase may be due to better treatment planning systems and algorithms. It may also be to better IMRT planning and more experience with these phantoms. In the course of ten years the phantom program has grown in phantom types and amount of each. This is correlated with the number of advanced technology clinical trials groups requiring the phantom for one of their credentialed requirements. d. b. Heart Anterior Right a. Left Primary PTV e. c. After the plan is created the institution is asked to perform their customary patient specific QA measurements. Next the phantom is positioned on the treatment couch and the treatment is delivered. After delivery, the water is drained and the phantom is returned to the RPC. Cord Posterior Figure 3. a. Lung phantom, b. axial film that was used for gamma analysis, c. coronal plane from the treatment planning system, d. gamma analysis, e. CT image. Once the phantom is returned, TLD are allowed to fade 14 days before being read. After the TLD and the film are read, dose distributions are compared to the institution’s electronically submitted treatment plan dosimetry data. TLD results, profiles through the PTV, and gamma analysis are report to the institution. Conclusions: The RPC’s QA phantom program has been an effective and responsive QA tool for assessing the use of advanced technologies in NCI sponsored clinical trials. The RPC has been efficient in its mailing of phantoms, and analyzing and reporting results. Support: Work supported by PHS grant CA10953 and CA081647 (NCI, DHHS).