Inspecting the Laboratory`s Quality Program
advertisement

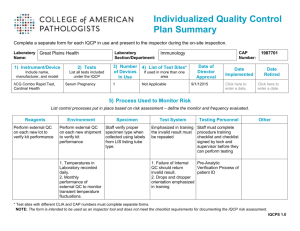
Your Quality Improvement Plan Gerald A. Hoeltge, MD Special Commissioner for Complaint Investigations, PTES Oversight & Equivalency Determinations CAP Laboratory Accreditation Program Objectives To clarify the checklist differences between quality improvement and quality control To identify aspects of laboratory service pivotal to patient care To help choose those indicators to monitor quality improvement 6 questions 13 questions on quality improvement We will point out the new questions. 13 questions on quality improvement QI is central to accreditation Standard III – – – – Quality Control Proficiency Testing Instrument Maintenance Quality Improvement / Performance Improvement Most checklist questions are Phase 2 Featured in the new individual grading report Grading Summary (partial example) Distribution of Performance by Peer Group Personnel and safety LAB BGS STD EXC EXC 8.2% 51.0% 40.8% 83.0% 12.6% Technical processesSTD 4.3% Quality improvement EXC 19.1% 0.0% 80.9% Proficiency testing STD 7.4% 87.0% 5.6% LAB = laboratory performance BGS = below peer group standard STD = standard EXC = excellent Standard III The Director shall... – monitor and evaluate quality and appropriateness – address systematic problems – participate in institutional QI programs and outcome studies – direct efforts to continuing improvement in quality Checklist questions on quality INFORMATION MANAGEMENT LAB INFORMATION SYSTEMS SPECIMEN MANAGEMENT REPORTING INTERPRETATION TESTING/REVIEW PROCESSING SPECIMEN TRANSPORT SPECIMEN COLLECTION 0 10 20 30 40 after Nevalainen DE, Berte LM. CAP Today, March, 1997 50 60 70 Why have a quality plan? No different than EOP, safety plan, FVPM statement Its purpose is not to please the inspection team, but the inspector should use it as a tool to make the inspection more efficient. Phase 2 Does the quality improvement program follow a written plan? Was it approved by the Director? Is it comprehensive? Is it structured? Sahney’s Second Law of Quality Progress: Qp = E x 2 M E = employees’ commitment to quality M = management’s commitment to quality “Approved by the Director” Evidence that the director is involved Responsibility under Standard I: “Assume responsibility for implementation of the quality improvement plan. The director and professional laboratory personnel must participate as members of the various quality improvement committees of the institution.” Quality is still everyone's responsibility! The best labs are those with a culture of quality. ISO 9000 series 1. management responsibility 2. quality system 3. contract review 4. design control 5. document control 6. purchasing 7. purchaser-supplied product 8. product identification and traceability 9. process control 10. inspection and testing 11. inspection, measuring and test equipment 12. inspection and test status 13. control of nonconforming product 14. corrective action 15. handling storage, pack aging and delivery 16. quality records 17. internal quality audits 18. training 19. servicing 20. statistical techniques ISO DIS 15189 organization and management quality management system document control referral of examinations to other laboratories external services and supplies control of nonconformities consultative services and resolution of complaints preventive actions corrective actions quality and technical records internal audits management review ISO/TC 212/WG 1: “Quality Management in the Medical Laboratory,” Draft International Standard NCCLS Guideline GP-22 “Continuous Quality Improvement” Team management Plans management Improvement manage ment Selecting an improvement process Systematic improvement in the path of workflow The TQM interface operating systems GP26 Q U A L I T Y S Y S T E M S 1.patient assessment 2.test requests 3.specimen collection 4.specimen transport 5.specimen receipt 6.testing and review 6.lab interpretation 7.results reporting 8.post-test specimen management 9.laboratory information system 10. interpretation and consultation quality system essentials 1.quality program organization 2.personnel selection, training, education 3.equipment 4.purchasing and inventory 5.process control 6.. documents and records 7.occurrence management 8.internal assessment 9.process improvement 10. service, satisfaction after NCCLS GP26-P (1998) “Quality System Model for Healthcare” GP26 Q U A L I T Y S Y S T E M S • organization • personnel • equipment • purchasing • process control interpretation patient • records and consultation assessment • occurrence management • internal assessment • process improvement • customer satisfaction Important aspects of care critical to patient care high volume experientially problematic Phase 1 Does the lab follow the plan? Has the QI plan been implemented as designed? refer to the design document. based upon the document that was in effect ~ 12 months prior to the inspection. NEW Monitoring and Evaluation design measure improve assess Shewhart Cycle plan act do check Phase 2 Benchmarks of performance? – Are key indicators of quality monitored and evaluated for opportunities for improvement? Are the chosen indicators being measured against a benchmark such as a practice guideline, Q-Probe data, published references, or trend analysis? Sufficient measure indicators for the laboratory’s scope of care? Caution! Measurement does not improve quality. Documentation must not become its own goal. Organizations may decouple related processes. Rigid external benchmarks stifle quality improvement. Phase 1 Pre-analytic measures? Do the chosen indicators include measures of preanalytic variation appropriate to the laboratory’s scope of care? – transmission of physicians’ orders – shipment of provider-collected samples – requisition accuracy – phlebotomy statistics – specimen acceptability rates NEW Phase 1 Post-analytic measures Do the chosen indicators include measures of postanalytic variation appropriate to the laboratory’s scope of care? – reflexive testing – concordance of analytic data – off-site printing and posting of lab reports – report readability NEW Phase 2 Adequate design? Is design the QI program comprehensive? – Each section of the laboratory, e.g., chemistry, transfusion medicine, anatomic pathology, microbiology, hematology, etc. – The program must include and integrate all aspects of the lab’s scope of care, such as inpatient and outpatient services, reference laboratory services, satellite and point-of- care testing, and consultative services. Typical table of organization in 2000: • Anatomic Pathology • • • • • – Surgical Pathology – Cytopathology – Autopsy Pathology Specimen Procurement and Processing Lab Automated Laboratory Manual Testing – Microbiology – Immunology – Special Chemistry – Cytogenetics Laboratory Information Systems Outpatient Clinic Labs Phase 2 Problem resolution Is there a systematic program to identify and correct problems that may interfere with patient care services? – Follow a plan? – Statistics available? – All problems recorded? – Resolution documented? NEW Mistakes are opportunities. Phase 1 Actions taken? Are appropriate actions taken whenever opportunities for improvement are identified? Each opportunity should eventuate in an appropriate action. Records should be organized in such a way as to facilitate identification of opportunities to improve care. NEW Phase 1 Customer satisfaction Have the referring physicians’ and patients’ satisfaction with the laboratory service been measured within the past 2 years? physicians direct indirect patients telephone survey questionnaire referral statistics waiting times (examples) NEW Phase 2 Annual appraisal Is the QI program appraised at least annually for effectiveness? – – – – – by the director or qualified designee new and redesigned activities follow-up and problem resolution address recurrent problems revise summary document when indicated NEW Phase 1 Charts and graphs? Are graphical tools used to communicate quality findings effectively? Examples: • Pareto charts • cause-and-effect (fishbone) diagrams • frequency histograms • trend graphs • force-field analyses • flow charts NEW Phase 2 Clinical relevancy of QI program Is a physician responsible for ensuring that the program is coordinated with those of the medical, surgical, and nursing services? The intent is to consider quality improvement of patient care services a medical process. One or more improvement activities were chosen after consultation with the medical staff. Phase 1 Is quality improving? Is there evidence that the laboratory’s quality has improved in the preceding 2 years? NEW QI records may be peripheralized or centralized. QI records may be peripheralized or centralized. If records are distributed in many locations, summaries of the content should be available at the central facility. How does the CAP inspector evaluate the QI program? Requirements are directive but not prescriptive. There is an opportunity for post-inspection dialog. The goal is laboratory improvement. The CAP inspector should be aware of the facility’s needs Where is the lab in its “QI evolution”? What is are the characteristics of the lab’s external and internal markets? What are the lab’s customers demanding? The program must be fair to all other applicants. Prepare for the inspection! Be familiar with the Standards and the checklist. Anticipate your inspector’s questions. Organize your QI records into a presentation. Have examples of QI reports. Show how they’re used to improve quality. Don’t be passive! Take the initiative! Show enthusiasm! Summary A systematic program is essential. Your documentation should be complete. Emphasize clinical relevance. This may be the most important part of the entire inspection!