Primary outcome - Foundation for the National Institutes of Health

Maternal-Fetal Surgery for
Myelomeningocele
Catherine Y Spong, MD
Pregnancy and Perinatology Branch, NICHD
National Institutes of Health
• MOMS Centers
– The Children’s Hospital of
Philadelphia
– University of California-
San Francisco
– Vanderbilt University
Medical Center
• Coordinating Center
– The George Washington
University Biostatistics
Center
• NICHD
– Pregnancy & Perinatology
Branch
Management of
Myelomeningocele Study
(MOMS)
•
Aim : To compare the safety and efficacy of in utero repair of open neural tube defects with standard postnatal repair
•
Intervention: Unmasked randomized clinical trial
•
Outcome evaluation by blinded independent investigators
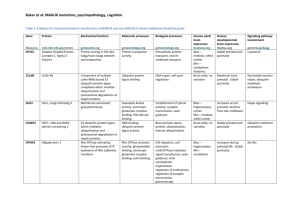
Myelomeningocele
• Most common & severe CNS congenital anomaly
• Affecting ~ 1500 fetuses in US annually
• Significant morbidity and mortality
• Life-long disabilities
• Severity correlated with levels of the spinal cord lesion
Complications
Hydrocephalus
Need for ventriculoperitoneal shunting
Motor and cognitive impairments
Bladder and bowel incontinence
Social and emotional challenges
Myelomeningocele: Fetal Sheep Model
In utero coverage of MMC
Rescues neurologic function at birth
Without prenatal coverage With prenatal coverage
Human Fetal Myelomeningocele Repair
Goal of the Trial
To compare the safety and efficacy of in utero repair of myelomeningocele (MMC) with that of the standard postnatal repair
Inclusion criteria
• Singleton
• Upper MMC boundary at T1-S1
• Evidence of hindbrain herniation
• GA 19.0-25.9 weeks at randomization
• Normal karyotype
• US residency
• Maternal age >18 years
Major exclusion criteria
• Fetal anomaly unrelated to MMC
• Severe kyphosis
• Risk of PTB (short cervix, prior PTB)
• Placental abruption
• BMI >35
• Contraindication to prenatal surgery
Central Screening at Coordinating Center
Screening at Clinical Site (2 days)
Travel & lodging arranged
Mother and support person
Paid by MOMS center
Evaluation process
If requirements met, offered randomization
Comprehensive ultrasound
MRI of fetus
Fetal echocardiogram
Psychological testing
Meetings with evaluations team
Fetal surgeon
Neurosurgeon
Nurse
Neonatologist
Social worker
Anesthesiologist
Perinatologist
Randomization to Neonatal Discharge
Moms and infants go to assigned center
Prenatal group
Admitted to MOMS center
In utero repair
Remain near center until delivery
Deliver by CD
@ 37wks if undelivered
Postnatal group
Return home
Return at 37wks to MOMS center for delivery by CD
Postnatal closure within 48h
Primary Outcome (12 months)
• Death or need for ventricular decompressive shunting at 12 months defined by objective criteria
– If shunt placed without meeting criteria – qualifies as primary outcome
• Independent committee of neurosurgeons, blinded to treatment assignment, determines whether criteria have been met
Primary Outcome (30 months)
• A composite score from the Bayley Scales of
Infant Development MDI and the difference between the motor level and lesion level
• Evaluated by independent examiners blinded to treatment assignment
• Videotapes of physical exams reviewed by independent expert
Secondary Outcomes
• Gestational age at delivery
• Hindbrain herniation
• Difference between motor function and anatomic levels
• Ambulation
• Oligohydramnios
• Blood transfusion at delivery
• Placental abruption
• Pulmonary edema
• Hysterotomy site
• Bradycardia at fetal repair
L2 –S4 Myelomeningocele
T12 L1
L2
L3
Motor Impairment: Level of Spinal Cord Injury
Secondary Outcome:
Difference between motor function and anatomic levels
(Observed motor function) – (anatomic level)
(obs S1) – (anatomic L4) =
+
2 levels
(obs L2) – (anatomic L4) = - 2 levels
Demographics
Fetal gender female — no. (%)
Gest. age at randomization (wk)
Maternal age ( yr)
Black or African American
White
Married — no. (%)
Years of schooling — no. (%)
Body mass index at trial entry
Current smoker — no. (%)
Nullipara — no.(%)
Cervical length — mm
Prenatal n=78
35 (44.9)
23.6 ± 1.4
29.3 ± 5.3
1 (1.3)
73 (93.6)
73 (93.6)
14.8 ± 1.7
26.2 ± 3.7
6 (7.7)
33 (42.3)
38.9 ± 7.3
Postnatal n=80
51 (63.8)
23.9 ± 1.3
28.8 ± 4.9
1 (1.3)
74 (92.5)
74 (92.5)
15.0 ± 1.6
25.9 ± 3.9
4 (5.0)
36 (45.0)
39.7 ± 5.7
Demographics (cont’d)
Prenatal n=78
Lesion level by sonogram
Thoracic
L1-L2
L3-L4
L5-S1
Lesion level L3 or lower
Clubfoot by ultrasound
Postnatal n=80
4 (5.1) 3 (3.8)
21 (26.9) 10 (12.5)
30 (38.5) 45 (56.3)
23 (29.5) 22 (27.5)
53 (67.9) 67 (83.8)
20 (25.6) 15 (18.8)
MOMS: Primary Outcome (12 mo)
death or need for shunt
Primary outcome
Prenatal n=78
53(68%)
Death before shunt
Shunt criteria met
2( 3%)
51(65%)
Shunt placed without criteria 0
Postnatal P value n=80 RR (95%CI)
78(98%) <0.001
0.70(0.58-0.84)
0
74(92%)
4( 5%)
Placement of shunt 31(40%)
Two perinatal deaths in each group:
Prenatal: IUFD at 26wks, NND at 23 wks
Postnatal: NND with severe symptoms of Chiari II
66(82%) <0.001
0.48(0.36-0.64)
MOMS: Primary Outcome (30 mo)
Prenatal n=64
Postnatal n=70
P value
148.6+57.5
122.6+57.2
0.007
Primary outcome
Bayley MDI
Difference between
89.7+14.0
0.58+1.94
motor function & anatomic level
87.3+18.4
-0.69+1.99
0.53
0.001
Secondary Outcome: Hindbrain Herniation
(12 months)
Secondary Outcome:
Difference between motor function and anatomic levels
P=0.002
better better worse worse
Secondary Outcome: Ambulation
Walking independently
Prenatal n=64
Postnatal P value n=70 RR (95%CI)
26/62(42%) 14/67(21%) 0.01
2.01(1.16-3.48)
Walking status
None
Orthotics/devices
Walking independently
18/62(29%) 29/67(43%)
18/62(29%) 24/67(36%)
26/62(42%) 14/67(21%)
0.03
Maternal Outcomes
Chorioamniotic membrane separation
Pulmonary edema
Modified biophysical profile < 8
Oligohydramnios
Placental abruption
Chorioamnionitis
Blood transf. at deliv
Prenatal n=78
20 (25.6)
5 (6.4)
13 (16.7)
16 (20.5)
5 (6.4)
2 (2.6)
7(9.0)
Postnatal n=80
0 (0.0)
0 (0.0)
6(7.5)
3 (3.8)
0(0.0)
0 (0.0)
1 (1.3)
RR
(95% CI)
—
—
2.22
(0.89
– 5.55)
5.47
(1.66-18.04)
—
—
7.18
(0.90-57.01)
P
<0.001
0.03
0.08
0.001
0.03
0.24
0.03
Maternal Outcome:
Hysterotomy site
Intact, well-healed
Very thin
Area of dehiscence
Complete dehiscence
Prenatal n=76
49 (64.5)
19 (25.0)
7 (9.2)
1 (1.3)
35.5%
Fetal and Neonatal Outcomes
Bradycardia at repair
Perinatal death
GA at birth
< 30 wks
35-36 weeks
>=37 weeks
Prenatal
N=78
8 (10.3)
2 (2.6)
34.1
± 3.1
10 (12.8)
26 (33.3)
26 (33.3)
16 (20.5)
Postnatal
N=80
0
2 (2.5)
37.3
± 1.1
0 (0.0)
4 (5.0)
8 (10.0)
68 (85.0)
RR (95% CI)
15%
P
1.03 (0.14-7.10)
0.003
1.00
<0.001
Neonatal Outcomes (cont’d)
Birth weight (g)
Dehiscence at repair site
RDS
Sepsis — no. %
Prenatal
N=78
Postnatal
N=80
2383 ± 688 3039 ± 469
10 (12.8)
16 (20.8)
4 (5.2)
5 (6.3)
5 (6.3)
1 (1.3)
RR
(95% CI)
P
2.05
(0.73-5.73)
3.32
(1.28-8.63)
4.16
(0.48-36.36)
<0.001
0.16
0.008
0.20
Summary
• Prenatal surgery for myelomeningocele reduces the need for a shunt or death and improves motor outcomes at 30 months but is associated with maternal and fetal risks
Summary
• Prenatal surgery is associated with other favorable secondary outcomes:
– Reduces hindbrain herniation at 12 months
No evidence of herniation in 36% vs 4%
– Doubles ability to walk without orthotics
42% vs 21%
– More likely to have a level of function that was two or more levels better than expected according to anatomic levels
32% vs 12%
Summary
• Prenatal surgery associated with maternal and fetal risks
– Preterm birth: 80% vs 15%
• RDS in 21% vs 6%
– Bradycardia
– Oligohydramnios
– Placental abruption
– Transfusion at delivery
– Uterine dehiscence at surgical site (35%)
Many thanks to:
• Radiology Review committee
: Dorothy Bulas, M.D.,
Charles Fitz, M.D. and Gilbert Vezina, M.D.
• Shunt Outcome Review Committee
: D. Douglas Cochrane, M.D., James
Drake, M.D., John Kestle, M.D. and Jerry Oakes, M.D.
• Pediatrician and psychologist examiners : Alex Espinosa, M.D., Julia
Hayes, M.D., Elizabeth Higley, Ph.D., Rita Jeremy, Ph.D., Rowena
Korobkin, M.D., David Kube, M.D., Leanne Pollard, Jonathan Rowland,
Elizabeth Saslow, Ph.D. and Toni Whitaker, M.D.
• Training and QA monitoring : Mario Petersen, M.D., Melissa Fallone,
Ph.D., Theresa Leach, M.Ed. and Susan Anderson,M.D.
• The Data and Safety Monitoring Committee
: George Macones, M.D.,
Michael Ross, M.D., Donald Stablein, Ph.D., Alessandro Ghidini, M.D.,
Michele Prince, MS, C.G.C., Barbara Schmidt, M.D., Antoine Khoury,
M.D., Sonya Oppenheimer, M.D., John McLaughlin, M.D., Reverend
Phillip Cato, Ph.D., Kellie Murphy, M.D., M.Sc., Dale Phelps, M.D.,
Keith Aronyk, M.D., William Hay, Jr., M.D., Mary E. Hannah, M.D.,
M.Sc., Jeremy Sugarman, M.D.
And at the sites, many thanks to
• The Children’s Hospital of Philadelphia, Philadelphia, PA – Alan Flake, M.D.,
Holly Hedrick, M.D., Jamie Koh, R.N., M.S.N., Jack Rychik, M.D., David Cohen,
M.D., Natalie Rintoul, M.D., Beverly Coleman, M.D., Patrick Pasquariello, M.D.,
Enrico Danzer, M.D., Larissa Bilaniuk, M.D., Martha Hudson, M.S.W., Michael
Carr, M.D., Ph.D., Michael Bebbington, M.D., M.H.Sc., Julie Moldenhauer, M.D., and R. Douglas Wilson, M.D.
•
University of California San Francisco, San Francisco, CA
– Michael Harrison,
M.D., Hanmin Lee, M.D., Larry Rand, M.D., Tamara Ryan, R.N., Cindy Lazzaretti,
R.N., Rachel Perry, R.N., Stephanie Berman, L.C.S.W., Vicki Feldstein, M.D., Ruth
Goldstein, M.D., Peter Callen, M.D., Orit Glenn, M.D., Larry Baskin, M.D., Mark
Rosen, M.D., Charles Cauldwell, Ph.D., M.D., and Vilma Zarate, Ph.D.
•
Vanderbilt University Medical Center, Nashville, TN
– Katharine Wenstrom, M.D.,
Lisa Trusler, R.N., M.S.N., Joseph Bruner, M.D., Bill Walsh, M.D., Edmund Yang,
M.D., Ph.D., Ann Kavanaugh-McHugh, M.D., Tracy Perry, Jennifer Anderson,
R.N., Mark Bliton, Ph.D. and Denise Pepin, M.S.W., L.C.S.W.
•
The George Washington University Biostatistics Center, Washington, DC
– Jessica
Ratay, M.S., C.G.C., Erin Greenbaum Musok, M.A., Kristen Holloway, Catherine
Shaer, M.D., Shanika Gregory, Julia Zachary, Lucy Leuchtenburg, Jeremy Drehmer,
M.P.H. and Megan Mitchell, M.P.H.
• The Eunice Kennedy Shriver National Institute of Child Health and Human
Development, Bethesda, MD
– Susan Tolivaisa, Nancy Chescheir, M.D. and Marian
Willinger, Ph.D.
• MOMS Centers
– The Children’s Hospital Of
Philadelphia
–
University Of California-
San Francisco
– Vanderbilt University
Medical Center
• Coordinating Center
– The George Washington
University Biostatistics
Center
• NICHD
– Pregnancy and
Perinatology Branch
Thanks to:
•
The women, their children and families who have taken part and continue to take part in the MOMS trial
•
The fetal therapy community
•
The perinatal community
• The Society for Maternal Fetal
Medicine