Formulating a question for systematic reviews
advertisement

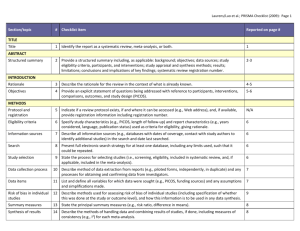
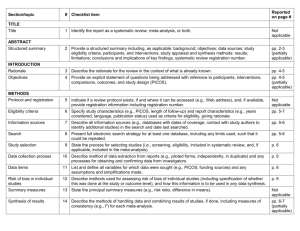
Formulating a question for systematic reviews Jenny Basford, Systematic Reviews Support Librarian mEsh j.basford@qmul.ac.uk The systematic review process Formulate research question Further selection of primary studies using inclusion criteria Extract data Design search strategy Write protocol Retrieve papers Quality Search bibliographic databases Identify possible papers from titles/abstracts Synthesis appraisal Formulate research / policy conclusions Learning outcomes • To understand the importance and purpose of setting out a well-formulated research question • To be aware of how bias can impact upon a systematic review from the outset • To be able to develop a clear review question Introduction: formulating a review question • A statement of what you are going to do and how you will do it • Gives a clear pathway to follow for the rest of the review • Thus reduces bias and error Creating a review question • Comprised of two parts: – ‘Free form’ question – Structured question Free-form question • Defined by simple language • Sometimes very vague • Describes the query that you are interested in – e.g. what effect does statin use have on pregnancy? Creating a structured research question: PICO(S) • • • • • Populations Interventions Comparators Outcomes Study design Populations • The group of participants/patients of concern to the reviewer • e.g. ‘all children under 16 years’; ‘men with history of heart conditions’ Interventions • Actions/exposures • e.g. treatments, social or educational interventions, risk factors, tests, drugs, surgical techniques • Refined by dosage/duration • Can be broad, e.g. ‘dietary supplement’ or specific, e.g. ‘Vitamin D, Cholecalciferol’ Comparators • If including comparative studies: don’t always have this • Similar definition as intervention • Comparison can be: no intervention, placebo, current standard practice or an active comparator: e.g. comparing accuracy of ultrasound vs. MRI scan in diagnosis of adenomyosis Outcomes • Clinical changes in health state, e.g. morbidity, mortality, survival • Health resource use • Quality of life • Behaviour Study designs • ‘major role in determining the reliability of the results’ (CRD) • RCTs usually the study design of choice for effectiveness reviews • Scoping search will help you decide whether to limit by study type • Depends entirely on the nature of your topic ‘Free form’ review question To assess the impact of statin use in pregnant women upon their unborn child Structured review question: PICOS for statins in pregnancy Population: pregnant women Intervention: statins Comparator: none Outcome: congenital malformations in the child Study design: RCTs Conclusion • Spending time on clearly defining your question at the beginning will help your review by providing a pathway and reducing bias • It will ensure everyone on the review is asking the same question