Rivaroxaban - NHS West Suffolk Clinical Commissioning Group
advertisement
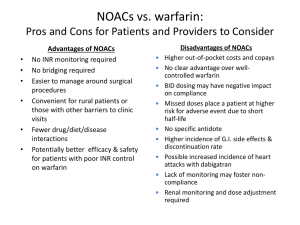
New Oral Anticoagulants (NOACs) Dabigatran and Rivaroxaban for the prevention of stroke and systemic embolism in nonvalvular atrial fibrillation Dr Dipti Chitnavis/ Dr Claire Hughes Consultants in Haematology West Suffolk Hospital Topics • Trials Summary • Trials Issues • Advantages and Disadvantages of NOACs compared to Warfarin • Effect on Coagulation Tests • Cardioversion/ Invasive Procedures • Management of Bleeding Trial data: RE-LY and ROCKET AF RE-LY: Randomized Evaluation of Long Term Anticoagulant Therapy Comparing the Efficacy and Safety of Two Blinded Doses of Dabigatran Etexilate With Open Label Warfarin for the Prevention of Stroke and Systemic Embolism in Patients With Non-valvular Atrial Fibrillation: Prospective, Multi-centre, Parallel-group, Noninferiority Trial RE-LY • Evaluated the non-inferiority of two doses of dabigatran compared with warfarin in people with AF who were at moderate to high risk of stroke • Primary efficacy endpoint was incidence of stroke (including haemorrhagic) and systemic embolism • Primary safety endpoint was major bleeding RE-LY • Lower dose dabigatran (110mg twice daily) found to be non-inferior to warfarin at reducing the risk of stroke and systemic embolism in people with AF • Higher dose dabigatran (150mg twice daily) found to be statistically significantly more effective than warfarin RE-LY • Mean rates for major bleeding: 2.71% per year for low dose dabigatran 3.11% per year for high dose dabigatran 3.36% per year for warfarin • Whereas lower-dose dabigatran was associated with a reduced risk of major bleeding, there were no significant differences between higher-dose dabigatran and warfarin in this respect RE-LY •Dabigatran thus demonstrated superiority to warfarin in preventing strokes, particularly haemorrhagic strokes, in people with AF who are at moderate or high risk of strokes. This finding, taken together with no greater risk of major bleeding, suggests a possible role as an alternative to warfarin in such patients. ROCKET AF: A Prospective, Randomized, Double-Blind, Parallel-Group, Multicenter, Non-inferiority Study Comparing the Efficacy and Safety of Rivaroxaban With Warfarin for the Prevention of Stroke and Non-Central Nervous System Systemic Embolism in Subjects With NonValvular Atrial Fibrillation ROCKET AF • Designed to determine whether rivaroxaban was non-inferior to dose adjusted warfarin (target INR of 2.0 -3.0) in preventing stroke or systemic embolism among patients with non-valvular atrial fibrillation • Primary efficacy endpoint was composite event of stroke and systemic embolism • Primary safety endpoint was composite event of major/non-major clinically relevant bleeding ROCKET AF • In the per-protocol treatment group, the event rates for stroke and systemic embolism were: 1.7% per year in the rivaroxaban group 2.2% per year in the warfarin group • In the intention to treat population as part of sensitivity analysis, the event rates for stroke and systemic embolism were: 2.1% per year for rivaroxaban 2.4% per year for warfarin ROCKET AF • Clinically relevant bleeding event rates were: 14.9% per year in the rivaroxaban group 14.5% per year in the warfarin group • Intracranial haemorrhage occurred less frequently with rivaroxaban as did fatal bleeding. ROCKET AF •Rivaroxaban was thus shown to be non-inferior to warfarin in preventing strokes or systemic embolism in people with atrial fibrillation who are at moderate to high risk for a stroke, while demonstrating a comparable risk of major and non-major clinically significant bleeding. Intracranial haemorrhage occurred less frequently than with warfarin, but the incidence of gastrointestinal bleeding increased. Stroke prevention efficacy Dabigatran low dose (110mg twice daily) non-inferior to warfarin at reducing the risk of stroke and systemic embolism in people with AF Dabigatran standard dose (150mg twice daily) statistically significantly more effective in preventing stroke, particularly haemorrhagic stroke, in people with AF with a moderate/ high risk of stroke, compared to warfarin. The number needed to treat to prevent one systemic embolism or stroke per year is 172 Rivaroxaban non-inferior to warfarin at reducing the risk of stroke and systemic embolism in people with AF Trials Issues RE-LY (Dabigatran) ROCKET (Rivaroxaban) • TTR Warfarin (INR 2-3) 64% (WSH TTR 71.95%) • Av. Age 71yrs • Rx discontinuation (Dabigatran>Warfarin) • Exclusions: Previous Hx of GI bleed • TTR Warfarin (INR 2-3) 55% • Av. Age 73yrs • Annual discontinuation rate (Rivaroxaban 23.7%; Warfarin 22.2%) Advantages & Disadvantages of NOACs & Comparison to Warfarin • Safety data NOACs are new drugs with a lack of long-term safety and tolerability data; rivaroxaban is a ‘black triangle’ drug Warfarin used for many years; longterm safety data available • Antidote NOACs have no specific antidote Warfarin has specific antidote • Half-life Dabigatran: 12-14 hours (normal renal function) Rivaroxaban: 5-9 hours (young); 11-13 hours (elderly) Warfarin: 40 hours Risk of NOAC treatment failure unless compliance is consistently good; compliance is critical as protection from stroke will be lost with omission of only one dose, compared to warfarin NOACs may be suitable for patients who are compliant with warfarin treatment, and yet are not well controlled (TTR<60%) • Swallowing difficulty Dabigatran: cannot be crushed or administered by nasogastric tube Rivaroxaban: can be crushed Warfarin: can be crushed; oral solution available • Suitability for monitored dosage systems (MDS) Dabigatran and warfarin: not suitable Rivaroxaban: suitable Risk of bleeding Dabigatran: less major bleeding on lower dose compared to warfarin Rivaroxaban: more nose bleeds and haematuria, less intracranial haemorrhage, less fatal bleeding compared to warfarin • Risk of GI bleeding Dabigatran standard dose (150mg twice daily) causes a higher risk of GI bleeding, and at both doses is associated with increased GI side effects, compared to warfarin Rivaroxaban causes a higher risk of GI bleeding, and is associated with increased major GI bleeding and increased GI side effects, compared to warfarin Renal impairment – Dabigatran: avoid if creatinine clearance <30ml/min; caution advised if eGFR 40-50 – Rivaroxaban: avoid if creatinine clearance <15ml/min; caution if creatinine clearance 1529ml/min; caution advised if eGFR 40-50 – Warfarin is not contra-indicated in renal impairment Hepatic impairment • Dabigatran: avoid in liver disease, hepatic impairment expected to impact on survival, elevated liver enzymes >2 upper limit of normal • Rivaroxaban: contraindicated in hepatic disease associated with coagulopathy and clinically significant bleeding risk, including Child Pugh B and C • Warfarin: caution with unstable liver function • Risk of MI The standard dose of dabigatran (150mg twice a day) was associated with a small but significant increase in MI; for every 476 people on dabigatran standard dose (150mg twice daily), one additional MI was observed No. needed to harm (NNH) 476 vs (NNT 172) Avoid dabigatran if high risk of coronary heart disease Cardioversion Patients can stay on dabigatran and warfarin while being cardioverted No data on rivaroxaban in cardioversion Invasive procedures-elective • Discontinuation may be required depending on bleeding risk/ type of procedure. • Timing of discontinuation depends on CrCl • SPCs provide specific information Unplanned surgery Problems if patients on NOACs require unplanned surgery or procedures; omit dabigatran dose prior to the procedure; stop rivaroxaban 24 hours prior to intervention Patients requiring emergency surgery can have the anticoagulant effects of warfarin reversed with dried prothrombin complex and intravenous vitamin K1 Assessing anticoagulant effect No clearly defined mechanism by which to determine if NOACs are working effectively in individual patients making compliance difficult to assess For patients on warfarin, INR can be monitored to ensure patient is within therapeutic range and compliant with treatment Effects on coagulation tests Dabigatran Rivaroxaban • APTT and TT prolonged • PT prolonged NOT ACCURATE MEASURE OF ANTICOAGULATION (Do not use INR) NOT ACCURATE MEASURE OF ANTICOAGULATION • D-dimers lowered! • D-dimers lowered! • Haemoclot Thrombin Inhibition • TIMING of test! • Anti-Xa specific for Rivaroxaban • TIMING of test! Management of bleeding on NOACs General non-pharmacological measures • • • • • • • • • STOP antithrombotics Document timing and amount of last dose Consider renal/hepatic impairment Estimate half- life of drug Mechanical measures FBC, APTT/ PT/TT/Fibrinogen/Creat/LFT Quantitative lab test to assess anticoagulation Resusc measures Surgical measures Management of bleeding on NOACs -specific measures Dabigatran • Oral activated charcoal (if last dose in last 2 hours) • Haemodialysis/haemofiltration and charcoal haemoperfusion • APCC (FEIBA), PCC (Octaplex/Beriplex), or rVIIa if ongoing life-threatening Rivaroxaban • PCC (Octaplex/Beriplex), APCC (FEIBA) or rVIIa if ongoing life-threatening Advantages of NOACs • • • • Standard dosing No routine monitoring No food interaction Fewer drug interactions (Appendix 6 of the WSCCG Guidelines) • Monitored dosage system possible (Rivaroxaban) NPSA requirements for Warfarin (VKAs) & NOACs • • • • Counselling Alert Card Patient support Regular review of anticoagulation Key Points • Efficacy from trial data may not be applicable to each patient • Assess patient carefully for suitability for anticoagulation (falls risk?) • Compliance important with shorter half-lives • Assess cautions/contraindications of NOACs compared to warfarin (renal/hepatic/cardiac/bleeding history) • Routine coagulation tests are not reliable for NOACs • No antidote to date • Regular review • Long term safety