Multiple Myeloma: History,
Biology and Treatment
Marcelo C. Pasquini, MD, MS
Associate Professor of Medicine
Adult BMT Program
Heme-Malignancies
Division of Hematology/Oncology
Outline
• Historical Perspective
• Biology
• Treatment
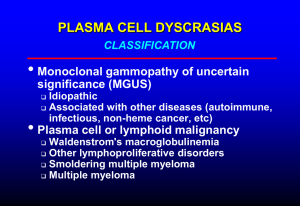
Multiple Myeloma
• Cancer of the immune system
– Antibody-producing cells called
plasma cells.
– Abnormal production of
antibodies
• Myeloma cells crowd out the
bone marrow and interfere
with the immune system and
the bones.
• Production of abnormal
antibodies, or M-protein, have
direct and indirect effects on
the blood, kidneys, and other
organs.
Durie. Concise Review of the Disease and Treatment Options: Multiple Myeloma. International Myeloma Foundation. 2011/2012 edition;
Multiple Myeloma Research Foundation. Multiple Myeloma Disease Overview. 2011.
Myeloma Early History
1844
First Case
Described
Sarah Newbury
“Mollites Ossium”
1873
Von Rustzky
“Multiple Myeloma”
1845
Case of
Pre History
Thomas McBean
Skeletal evidence Bence Jones
Protein
1898
Weber
X-Rays for
Diagnosis
1889
Prof. Otto Kahler
Detailed Description
of Symptoms
Kahler’s Disease
1939
Longsworth
Electrophoresis in myeloma
“Church Spire” peak
1900
Wright
Association
with plasma cells
in the bone marrow
Malignant Plasma Cells Produce a
Single Monoclonal Antibody
Types of Serum M-Protein
in Multiple Myeloma
Immunoglobulin
Constant
region
2% biclonal
Light chain
Κ or λ
Variable
region
7% none
16% light
chain only
52% IgG
Heavy chain
IgG, IgA, IgD,
or IgM
21% IgA
0.5% IgM
2% IgD
Durie. Concise Review of the Disease and Treatment Options: Multiple Myeloma.
International Myeloma Foundation. 2011/2012 edition; Kyle. Mayo Clin Proc. 2003;78:21.
Normal vs Myeloma
IgG κ
IgG L
IgA κ
IgA L
IgD κ
IgD L
IgE κ
IgE L
IgM κ
IgM L
Normal
Lots of different types of
whole antibodies
IgG κ
IgG L
IgA κ
IgA L
IgD κ
IgD L
IgE κ
IgE L
IgM κ
IgM L
IgG κ
IgG κ
Myeloma
20th Annual Update in Primary
Care
Whole Ab and excess light
chains – and too many of both!
M-Protein, Bone Marrow Plasma Cells, and Organ
Impairment Define Plasma Cell Disorder
MGUS
Asymptomatic/
Symptomatic/
Smoldering MM
Active MM
M Protein
Present
Elevated
High
Bone Marrow
Plasma Cells
Low
Elevated
High
Organ/Tissue
Impairment
None
None
Present
Kyle. Leukemia. 2009;23:3.
Criteria for Symptomatic Myeloma
i.e Needs treatment for “cancer”
Criteria for Symptomatic MM (all 3 required)
1
≥ 10% monoclonal plasma cells in bone marrow
2
Monoclonal protein in serum and/or urine
3
Presence of end-organ damage (at least one of the below)
Calcium
Renal
Anemia
Bone
“Infections”
Serum calcium ≥11.5 mg/100 mL
Serum creatinine >1.73 mmol/L
Hb <10 g/100 mL or >2 g/100 mL below normal
Lytic lesions, severe osteopenia, pathologic fractures
Repetitive bacterial infections
Additional “soft signs” – Neuropathy, Osteoporosis, Frequent infections, Proteinuria
Modified from Palumbo. Leukemia. 2009;23:1716.
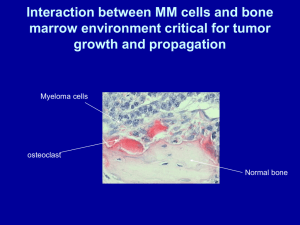
Importance of Interaction Between Plasma Cells
and Bone Marrow for Development of Myeloma
Palumbo A. and Anderson KC. New Engl J Med 2011;364:1046-1060
- Anemia
- Dysfunctional
humoral immunity
Correlation with
disease burden
Assessment of disease
response
Immune
dysregulation
Proliferation
Alterations
in the
Microenviroment
- Bone destruction
- Hypercalcemia
Multiple
Myeloma
Production
- Dysfunctional
humoral immunity
- Organ failure
- Hyperviscosity
Models of Cancer Development
Clonal Homogeneity
Inter-clonal Heterogeneity Intra-clonal Heterogeneity
Brioli A et al. British Journal of Haematology 2014
Evolutionary Biology of Cancer
Darwin’s Notebook
sketches
Brioli A et al. British Journal of Haematology 2014
Lohr JG et al Cancer Cell 2014
Clonal Architecture at Diagnosis and Relapse:
Clonal Tides Instead of Linear Evolution
Bahlis et al. Blood 2012;120:1077-1086
Kyle R.A. Blood 2008
Overall survival from diagnosis
of multiple myelomas.
2 years
3.5 years
4.6 years
Kumar S K et al. Blood 2008;111:2516-2520
©2008 by American Society of Hematology
Continued Improvement in Survival Since the
Introduction of Novel Agents
• 1,056 pts grouped into 2001–2005 and 2006–2010 cohorts
• Survival improved over time, particularly in pts aged > 65 years (p
= 0.001)
Proportion surviving
1.0
2001–
2005
2006–
2010
p
Median OS, yrs
4.6
NR
0.001
1-yr survival, %
83
90
Overall
48
66
> 65 yrs
31
56
0.001
< 65 yrs
63
73
NS
Survival
Diagnosed 2006–2010
0.8
0.6
5-yr estimated
OS, %
Diagnosed 2001–2005
0.4
0.2
0
0
3
2
1
4
Follow-up from diagnosis (yrs)
5
Kumar SK, et al. Blood. 2012;120:[abstract 3972]. Updated data presented at ASH 2012.
Classes of Drugs With Anti-MM Activity
Steroids
Immunomodulatory Agents
Proteasome
Inhibitors
Prednisone
Thalidomide
Bortezomib
Dexamethasone
Lenalidomide
Carfilzomib
Pomalidomide
Ixazomib
Oprozomib
Marizomib
CEP-18770
(Delanzomib)
Classes of Drugs With Anti-MM Activity
Cytotoxic CT
HDAC
inhibitors
mTOR
inhibitors
mAbs
Melphalan
Vorinostat
Perifosine
Elotuzumab
Cyclophosphamide
Panobinostat
BCNU
Bendamustine
Anthracyclines
PACE
DCEP
Daratumumab
Myeloma Treatment Roadmap
Induction
Consolidation
Maintenance
Brioli A et al. British Journal of Haematology 2014
Induction Therapy
• Initial disease de-bulking
• Reduction of the paraprotein
• Decreasing the intra-clonal heterogeneity
Combinations in the Upfront Treatment of MM
V or P– Bortezomib
Combination therapy incorporating
A - Adriamycin
T – Thalidomide
R – Revlimid ; C - Cyclophosphamide
Carf-RD
novel agents results in near 100% ORRs
21
Stewart AK, Richardson PG, San Miguel JF. Blood. 2009.
Consolidation
•
•
•
•
Transplantation ± consolidation therapy
Maximize disease control
Goal: to reach complete response or better
Further reduce inter-clonal heterogeneity
Autologous HCT vs. Chemotherapy for
Newly Diagnosed Myeloma
Attal M. N Engl J Med 1996; 335:97
Child J. N Engl J Med 2003; 348:1875
Indications for Hematopoietic Stem Cell
Transplants in the US, 2011
Allogeneic (Total N=7,892)
Autologous (Total N=12,047)
Number of Transplants
7000
6000
5000
4000
3000
2000
1000
0
Multiple
Myeloma
NHL
AML
ALL
MDS/
MPD
CML
Aplastic
Anemia
CLL
Other
Other
Non-Malig Cancer
Disease
HD
24
Overall Survival of
Autotransplantation in MM
Barlogie B, et al. J Clin Oncol. 2010;28(7):1209-1214.
Maintenance
• Long term treatment with an anti-myeloma
agent that is tolerable and effective
• Maximize disease control
• Prevent the inception of “new” subclones
CALGB 100104: Updated TTP
Estimated HR=0.51
(95% CI = 0.39 to 0.66),
146/229 events (64%) on placebo
104/231 events (45%) on lenalidomide
ITT Analysis with a median follow-up from transplant of ~48
CALGB 100104 IMW 2013
months p<0.001 Median TTP: 50 months versus 27 months with
follow up to January 7, 2013
86 of 128 non-progressing placebo patients receiving
lenalidomide at study un-blinding in Jan 2010
CALGB 100104: Updated OS
Estimated HR=0.61
(95% CI = 0.41 to 0.87)
69/229 (30%) deaths on placebo
47/231 (20%) deaths on lenalidomide
CALGB 100104 IMW 2013
follow up to January 7, 2013
ITT Analysis with a median follow-up from transplant of ~48
months. p= 0.008, Median OS: not reached versus 73 months
Palumbo ASCO 2013
R maintenance vs No maintenance
Overall survival
Progression-free survival
48% reduced risk of progression
38% reduced risk of death
Median PFS
R maint.
100
5-year OS
37 months
100
No maint. 26 months
R maint.
75%
No maint.
58%
75
75
50
50
25
25
HR 0.52, 95% CI 0.40-0.67, P <.0001
0
HR 0.62, 95% CI 0.42-0.93, P =.02
0
0
10
20
30
40
50
60
70
0
Months
10
20
30
40
Months
o
s
R, lenalidomide
50
60
70
Caveats with Continuous Treatment
• Does using all “active” drugs at once favors
the inception of resistant subclones?
R maintenance vs No maintenance
OS from relapse
PFS from diagnosis
100
100
Delayed clonal
evolution
75
Chemoresistant
relapse
75
R maint.
50
50
R maint.
25
Faster clonal
evolution
25
No maint.
HR 0.52, 95% CI 0.40-0.67, P <.0001
0
0
10
20
30
40
Months
R, lenalidomide
Chemosensitive
relapse
50
60
70
No maint.
HR 0.82, 95% CI 0.55-1.22, P =.32
0
0
10
20
30
Months
40
50
60
Caveats with Continuous Treatment
• Does using all “active” drugs at once favors
the inception of resistant subclones?
• What are the risks of ongoing therapy?
CALGB 100104: Second Primary Malignancies (SPMs)
SPM
Len Arm
Placebo
Arm
Placebo
Crossover
to Len
Cross over
Time on len
in months
ALL
AML
HL
MDS
NHL
1
5+1
1
1
0
0
0
0
0
1
1
0
0
1
0
32 mo
Total Heme
8+1=9 1
2
Breast
Carcinoid
Brain
GI
Gyn
Melanoma
Prostate
Thyroid
Renal
3
0
1
2
1
1
1
1
0
0
1
0
1
1
2
0
0
0
0
0
0
0
0
0
0
0
1
Total Solid Tumors
10
5
1
Basal Cell Ca
Squamous Cell Ca
2+3
2
1
2
0
1
Total Skin Cancers
4+3=7 3
1
6 mo
14 mo
4 mo
CALGB 100104: Event-Free Survival, Updated
Estimated HR=0.55
(95% CI = 0.43 to 0.70),
ITT Analysis: Events are Progressions, Deaths and SPM.
CALGB 100104 IMW 2013
151/229 (66%) placebo patients and 113/231 (49%) lenalidomide
follow up to January 7, 2013 patients have experienced events. Median EFS 27 and 47
months respectively p <0.001
Proposed model of second malignancies after multiple
myeloma.
Thomas A et al. Blood 2012;119:2731-2737
©2012 by American Society of Hematology
- Anemia
- Dysfunctional
humoral immunity
Correlation with
disease burden
Assessment of disease
response
Immune
dysregulation
Proliferation
Alterations
in the
Microenviroment
- Bone destruction
- Hypercalcemia
Multiple
Myeloma
Production
- Dysfunctional
humoral immunity
- Organ failure
- Hyperviscosity
Multiple Myeloma Treatment: Future
Perspective
• Myeloma now is a chronic disease
– Patients are living longer than ever
– Although mostly incurable
• Modified targeted therapy paradigm
– Risk adapted or molecular signature adapted
– Change from continuous to non-continuous treatment
when appropriate – response adapted treatment
• Newer treatments
– Antibodies
– Vaccines
– Cell therapies
Acknowledgements
MCW Myeloma Program
• Parameswaran Hari
• Carlos Arce-Lara
• Anita de Souza
Roswell Park Cancer Institute
• Philip McCarthy
• Theresa Hahn
Funding: R01-HL107213
U10 HL69294-12
MCW Hem Malignancies
• Ehab Atallah
• Karen-Sue Carlson
• Christopher Chitambar
• William Drobyski
• Mary Eapen
• Timothy Fenske
• Mehdi Hamadani
• Mary Horowitz
• Laura Michaels
• Doug Rizzo
• Wael Saber