Benefits of JAK2 Inhibitor Therapy: Why Do They Work in
advertisement

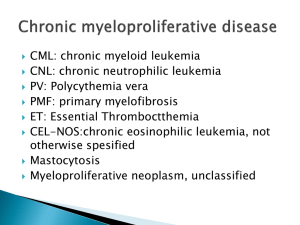
Benefits of JAK2 Inhibitor Therapy: Why Do They Work in Patients With and Without JAK2 Mutation Alessandro M. Vannucchi Section of Hematology, University of Florence, Italy JAK2 V617F is the Commonest Mutation Causing Abnormal JAK/STAT Signaling in MPN Survival Differentiation Proliferation Oncogenesis Vannucchi et al., CA Cancer J Clin. 2009; 59:171-91 A Myeloproliferative Disorder is Induced by JAK2 V617F in Mice PV MF Zaleska, Plos One 2006;e18 JAK2 inhibitors are not specific for the JAK2 V617F mutation Binding to receptors Chaperoning Stabilising at membrane FERM SH2 JH5 JH7 JH6 Inhibits basal activity Signaling through P transfer PseudoKinase Kinase ATP site Kinase site JH3 JH4 JH2 JH1 Activation loop V617F •JAK inhibitors target the ATP binding site of JAK2 at the tyrosine kinase V617 domain and not the pseudokinase domain • Therefore, both mutated and wild-type JAK2 are inhibited by JAK2 inhibitors James et al. Nature 2005; 434: 1144-8 ; Baxter et al. Lancet 2005; 365: 1054-61; Levine et al. Cancer Cell 2005; 7:387-97; Kralovics et al. NEJM. 2005: 352:1779-90 Inhibition of PV Progenitor Erythroid Differentiation by the JAK2 Inhibitor TG101348 Geron I et al, Cancer Cell, 13; 2008 321 - 330 Effects of Treatment with Ruxolitinib in a JAK2 V617F-Driven Murine Model Quintás-Cardama et al., Blood 2010;115:3109-3117. JAK1 and JAK2, or JAK2 Only, Inhibitors Drug Ruxolitinib/INC424 TG101348/SAR302503 JAK1 Other targets none known FLT3, Ret CYT387 JNK1, CDK2 CEP-701 FLT3, TrkA AZD1480 SB1518 LY2784544 Aurora A, TrkA, FGFr1 FLT3 na BMS911543 Verstovsek et al. N Engl J Med. 2010; 363:1117-27. Pardanani et al. JCO. 2011; online Jan 10. Pardanani et al. ASH Abstract 2010; Blood 2010; 116:460. Santos et al. Blood. 2010; 115:1131-6.La Fave LM, Trends Pharm Sciences 2012; 33:564-582. •Do they work? •If yes, Why? •Do they work? •If yes, Why? Spleen length, cm The Effects of Ruxolitinib on Spleen Size is Independent of JAK2 V617F Mutation JAK mutation POSITIVE; N = 33 22.5 20.0 17.5 15.0 12.5 10.0 7.5 5.0 2.5 0 0 JAK mutation NEGATIVE; N = 6 56 112 168 224 280 336 Time on Therapy (days) • In the Phase I/II study with TG101348/SAR302503, 8 of 59 pts were JAK2 wild-type • 3 of 4 pts who completed six cycles had >50% reduction of splenomegaly (CI per IWG-MRT) Verstovsek S et al. NEJM 2010; 363:1117-1127; Pardanani A et al, JCO 2011; 29:789-796 Effect of JAK2 V617F Mutation on the Proportion of Patients Obtaining a >35% Spleen reduction* • No significant difference in response rates was observed between patients with the JAK2V617F mutation compared with those without the mutation, although the trend was towards a greater response rate in JAK2 V617F mutated * By MRI Harrison C et al, ASH 2011; 279 The Effects of Ruxolitinib on Symptomatic Control Is Independent of JAK2 V617F Mutation Kiladjian JJ et al, ASCO 2012: 451A The Impact of Ruxolitinib on Survival Is Independent of JAK2 V617F Mutation Verstovsek S et al. ASH 2011, 378A •Do they work? •If yes, Why? Similarly Activated Signaling Pathways in JAK2 V617F Mutated and Wild-type MPN Cells Anand S et al. Blood 2011;118:1610-1621 Similar Inhibition of Signaling in JAK2 V617F and Wild-type patients with a Selective JAK2 inhibitor Anand S et al. Blood 2011;118:1610-1621 A Cytokine Storm in PMF Patients JAK2 V617F correlated Fold-increased over controls Tefferi A et al, JCO 2011;29:1356-1363 The Significance of JAK1 and JAK2 Inhibition Vannucchi AM, N Engl J Med. 2010; 363:1180-2. Ruxolitinib-Induced Normalisation of Inflammatory Cytokines in Phase I/II Trial Baseline, Patients with Myelofibrosis vs. Healthy Controls Patients with Myelofibrosis, Day 28 vs. Baseline This effect was observed regardless of JAK2 mutational status or MF subtype Verstovsek et al. N Engl J Med. 2010; 363:1117-27. Predicted Effects of JAK1 and JAK2 inhibition JAK2 inhibitors are not specific for mutated protein THUS they are effective regardless of the JAK2V617F mutated status JAK2wt (± JAK1) inhibition affects a variety of cytokine signaling pathways Concurrent Inhibition of JAK2wt might result in anemia and thrombocytopenia BAT Placebo 19.2 Anemia Thr’penia Ruxolitinib 43.2 1.3 12.9 31 Anemia • A marked reduction of pro-inflammatory cytokines was coincident with improvement in constitutional symptoms Thr’penia 42 7 8 % of patients Verstovsek S et al. N Engl J Med. 2010; 363:1117-27; Verstovsek S et al. NEJM 2012; 366:799-807; Harrison C et al. NEJM 2012; 366:787-98 Conclusions • Abnormal JAK/STAT signaling is a common pathogenetic mechanism in MPN cells independent of the JAK2 mutational status • Current JAK2 inhibitors are not specific for the mutated protein, and target the wild-type JAK2 as well • JAK2 inhibitors are similarly effective in JAK2 mutated and wild- type patients • Inhibition of wild-type JAK1 signaling contributes to the clinical efficacy of JAK1/JAK2 inhibitors