Part II
Screening of Human and Animal Sera from Egypt and Hong Kong
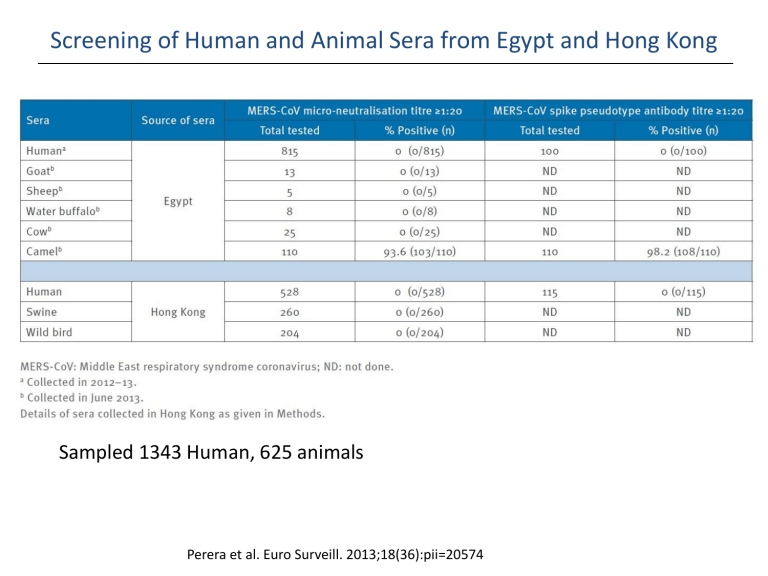
Sampled 1343 Human, 625 animals
Perera et al. Euro Surveill. 2013;18(36):pii=20574
Treatment
• SARS: definitive treatment regimen was not clearly established
• MERS CoV: Interferon alpha 2b + ribavirin in vitro 1
• Recent study in Macaque monkeys 2
• No clear data on human treatment
1 Falzarano D et al. Sci Rep 3 2013:1686
2 Falzarano D et al. Nature Medicine 2013 pub online 9/8/13
Vaccines
• SARS vaccines were in development
• Possible MERS vaccines:
– VLP/nanoparticles with S protein
– Constitutive expression of S protein in cell culture
• Developmental stages
Domestic Activities
• Case definition and guidance developed and disseminated
• 5 MMWRs published
• 3 health advisories sent to state/local health departments
• Investigating persons with travel link, severe respiratory illness
– 100 samples/31 states: all negative
• Serology developed
– MERS-CoV ELISA, MERS-CoV IFA and MERS-CoV MNt
• PCR diagnostics developed and distributed
CDC Case Definition
Patient Under Investigation (PUI)
A person with the following characteristics:
Fever (≥38 ° C, 100.4
° F) and pneumonia or acute respiratory distress syndrome (based on clinical or radiological evidence);
AND EITHER
History of travel from countries in or near the Arabian Peninsula within 14 days before symptom onset;
OR
Close contact with a symptomatic traveler who developed fever and acute respiratory illness (not necessarily pneumonia) within 14 days after traveling from countries in or near the Arabian Peninsula;
OR
Is a member of a cluster of patients with severe acute respiratory illness (e.g. fever and pneumonia requiring hospitalization) of unknown etiology in which MERS-CoV is being evaluated, in consultation with state and local health departments.
http://www.cdc.gov/coronavirus/mers/case-def.html
Epidemiology Tool Kit
Patient Under Investigation Form
Surveillance Plan: Phasic Response Tiers
Tier Description
0
No cases in the US, threat of importation, low risk of large-scale outbreak
1
Imported case(s) linked to exposure in an endemic country
National Surveillance Goals
Utilize key public health networks and current relevant surveillance programs for other pathogens or similar clinical syndromes
Geographically focused monitoring of routine surveillance networks
Potential expanded testing from inpatient and outpatient settings in affected area
Expanded testing from inpatient and outpatient settings in affected area
2
Secondary or tertiary cases linked to a primary case with known exposure in an endemic country
Enhance non-local surveillance by expanding case finding efforts or testing in existing programs
3
Index case in the US without endemic country exposure or efficient transmission of several generations from imported case
Possible modification of surveillance platforms and development of new surveillance
Active inpatient, outpatient and community setting surveillance in affected and priority areas
Passive, but broad case finding nationally
Domestic Deployment of CDC MERS-CoV rRT-PCR Assay by LRN
Approved for MERS-CoV testing (44)
Kits will be sent later (6)
Infection Prevention and Control Recommendations
• Standard, Contact and Airborne
Precautions
– N95 respirators if available
– Airborne infection isolation rooms
• Similar recommendations as SARS
– High mortality
– Human-to human transmission
– Unknown modes of transmission
– No vaccine or chemoprophylaxis
Infection Control: Checklists
• Prompt triage and testing of patients
• Prioritization of available isolation rooms
• Plans for appropriate cohorting of patients and personnel
• Training
• Sick leave policy











