Vitrasert Ganciclovir Intraocular Implant
advertisement

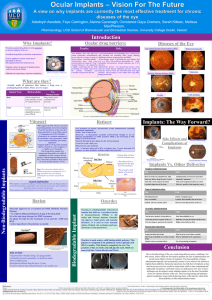
Vitrasert Ganciclovir Intraocular Implant • • • • • • • • • • • • • This is a reservoir style implant used for the delivery of the anti-viral drug ganciclovir to treat AIDs-related Cytomegalovirus (CMV) Retinitis which can lead to vision loss and blindness. CMV Retinitis is a chronic, sight-threatening, viral infection of the retina, the light sensing tissue in the back of the eye, which predominantly affects immunosuppressed individuals. The drug is embedded in a polymer-based system Vitrasert was the first non-biodegradable, intravitreal implant approved by the FDA in 1996 for the treatment of AIDs-related CMV retinitis which is estimated to affect between 15-40% of AIDS patients. Ganciclovir is a synthetic analogue of the nucleoside 2-deoxyguanosine and thus when incorporated into the elongating DNA strand it causes chain termination, preventing viral replication. Each implant holds 4.5-5mg of the drug which is released at a slow constant rate of approx. 1-1.5µg/hr over a 5-8 month time period. The implant is a 4mm device. Its structure consists of a compressed drug pellet core surrounded by a framework of two polymers; the impermeable polymer ethylene vinyl acetate (EVA) which controls the area through which the drug is released and the permeable polymer polyvinoyl alcohol (PVA) which regulates drug diffusion. The implant is surgically inserted into the posterior segment of the eye by making a 5-6mm incision into the pars plana which is part of the ciliary body. It is then fixed into place using scleral sutures. The wound is closed and a saline solution is injected to restore normal ocular pressure. The surgery typically takes an hour and is carried out under local anaesthetic. Complications from implantation are uncommon but include, endohpthalmitis(inflammation of the intraocular cavities i.e. the aqueous/vitreous humour), retinal detachment, cataract formation (clouding of the lens of the eye) and vitreous haemorrhage. Surgery is necessary to remove the device once depleted of the drug Ganciclovir ED50 for human CMV ranged from 0.2-3µg/ml Studies show that patients treated with the Vitrasert implant reduced the median time to CMV Retinitis disease progression compared to intravenous ganciclovir Vitrasert Ganciclovir Intraocular Implant • • • • • • • • • • • • Reservoir style implant used for the delivery of the anti-viral prodrug ganciclovir. Vitrasert was the first non-biodegradable, intravitreal implant approved by the FDA in 1996 for the treatment of AIDs-related Cytomegalovirus (CMV) Retinitis which is estimated to affect between 15-40% of AIDS patients.14 CMV Retinitis is a chronic, sight-threatening, infection of the retina caused by the cytomegalovirus (DNA virus of the herpes group), which predominantly affects immunosuppressed individuals and can lead to vision loss and blindness. Ganciclovir is a synthetic analogue of the nucleoside 2-deoxyguanosine, which causes chain termination, preventing replication. Each implant holds 4.5-5mg of the drug which is released at a slow constant rate of approx. 1-1.5µg/hr over 5-8 months. The 4mm device consists of a compressed drug pellet core is completely covered, except at its top surface, with the impermeable polymer ethylene vinyl acetate (EVA).This entire assembly is then coated by the permeable polymer, polyvinoyl alcohol (PVA) which regulates the drug’s diffusion. Water enters into the device dissolving the pellet and creating a saturated environment resulting in the sustained diffusion of the drug to the site of infection. The implant is surgically inserted into the posterior segment of the eye by making a 5-6mm scleral incision into the pars plana which is part of the ciliary body. It is then fixed into place using sutures. The wound is closed and a saline solution is injected to restore normal ocular pressure. The surgery typically takes an hour and is carried out under local anaesthetic. Surgery is necessary to remove the device once depleted of the drug and a new implant is then inserted. Most patients experience blurred vision which usually clears between 2-4 weeks after surgery. Though uncommon, complications include; endohpthalmitis, retinal detachment, cataract formation and vitreous haemorrhage. Advantages in comparison to I.V. ganciclovir include; reduced dosing frequency, enhanced drug efficacy, and reduced risk of toxicity. Phase III trial results from Bausch & Lomb of 188 AIDS patients with recently diagnosed CMV Retinitis showed that treatment with Vitrasert implant, significantly delayed the time to disease progression compared to treatment with intravenous ganciclovir. http://depts.washington.edu/hivaids/oit/case7/fi g7d.html http://tpx.sagepub.com/ content/36/1/49.full References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=18322 http://www.rxlist.com/vitrasert-drug.htm http://www.sciencedirect.com.eproxy.ucd.ie/science/article/pii/S0169409X06001645 http://emedicine.medscape.com/article/1227228-overview#a0104 http://www.retinatoday.com/Html%20Pages/0307/0307_feature_kuppermann.pdf http://www.iovs.org/content/45/8/2722.full.pdf https://mailattachment.googleusercontent.com/attachment/?ui=2&ik=f37170b2bc&view=att&th=13586d2d 5c3f686a&attid=0.1&disp=inline&realattid=f_gypyhscd0&safe=1&zw http://bjo.bmj.com/content/82/3/332.3.full http://bjo.bmj.com/content/83/11/1225.full http://www.oculist.net/downaton502/prof/ebook/duanes/pages/v3/v3c028a.html http://www.oculist.net/downaton502/prof/ebook/duanes/pages/v4/v4c045.html http://www.medicinenet.com/ganciclovir-intravitreal_implant/article.ht http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=18322 Henderly DE, Freeman WR, Causey DM et al: Cytomegalovirus retinitis and response to therapy with ganciclovir. Ophthalmology 94:425, 1987