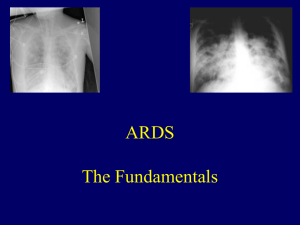
ards
advertisement

Acute Respiratory Distress Syndrome July 2014 OBJECTIVES • Acute Lung Injury (ALI) Acute Respiratory Distress Syndrome (ARDS) • Pathophysiology in ARDS • Therapy in ARDS EPIDEMIOLOGY • Accounts for 1-3% of admission to the PICU • Within ICUs, approximately 10 to 15 percent of admitted patients and up to 20 percent of mechanically ventilated patients meet criteria for ARDS. • Fackler et al. (1990) examined all PICU admissions at 40 institutions • 8000 admissions where 679 patients were identified as having ARDS (8.5%) ALI • A syndrome of acute and persistent lung inflammation with increased vascular permeability • Bilateral infiltrates consistent with pulmonary edema • A ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) between 201 and 300 mmHg (regardless of PEEP) • No clinical evidence of elevated left atrial pressure ARDS ARDS refers to the severe end of the spectrum of "acute lung injury". • Hypoxemia is worse (PaO2/FiO2 ≤200 mmHg) • Some studies suggest that SaO2/FiO2 may correlate with PaO2/FiO2 sufficiently to use as an estimate. • ARDS typically develops over 4 to 48 hours and persists for days to weeks. • Early pathological features of ARDS are generally described as diffuse alveolar damage (DAD). CAUSES OF ARDS • Sepsis • Aspiration • Cardiopulmonary bypass • Infectious pneumonia • Pulmonary contusion • Severe trauma • Multiple fractures • Surface burns • Following upper airway obstruction • Multiple blood transfusions • Following bone marrow transplantation • Drug overdose • Drug reaction • Near drowning • Embolism • Smoke inhalation • Miliary tuberculosis ALVEOLAR GAS EQUATION A-A GRADIENT • Difference in alveolar and arterial oxygen pressure. • A-a O2 Gradient = [ (FiO2) * (Atmospheric Pressure - H2O Pressure) - (PaCO2/0.8) ] PaO2 from ABG • Normal Gradient Estimate = (Age/4) + 4 ARDS-PATHOPHYSIOLOGY • Healthy lungs regulate the movement of fluid to maintain dry alveoli and minimal interstitial fluid. • This regulation is interrupted by lung injury, which results in excess fluid in both the interstitium and alveoli. • Inflammation results in increased permeability of pulmonary capillaries and alveoli, protein-rich pulmonary edema, surfactant depletion/inactivation • Loss of pulmonary vasomotor tone occurs due to refractory hypoxemia resulting in mild pulmonary hypertension. • Deranged balance between proinflammatory mediators (TNF alpha, IL-1 beta, IFN gamma, LT alpha, IL2, IL-8, IL-12, IL-18, Kinins, NO, GM-CSF, Chemokines, MIF, etc.) and anti-inflammatory mediators (sTNFR, sIL-1R, TGF beta, IL-4, IL6, IL-10, IL-11, IL-13, PGE2, GCSF,). ARDS Pathophysiology -Hyaline Membrane -Inflammatory Cascade -Protein-rich edema ARDS IS A HETEROGENEOUS PROCESS • Pathological features of ARDS involve 3 overlapping phases • 1. Inlammatory or exudative phase (0-7 days) • 2. Proliferative phase (7-21 days) • 3. Fibrotic phase (after 7-10 days) CONSEQUENCES • Impaired gas exchange — Ventilation-perfusion mismatching and physiologic shunting cause hypoxemia • Decreased lung compliance • Pulmonary hypertension — Pulmonary hypertension occurs in up to 25 percent of patients with ARDS who undergo mechanical ventilation. NORMAL LUNG DIFFUSE ALVEOLAR DAMAGE Histologic photograph or ARDS. Note inflammatory cells, hyaline membranes ARDS Diffuse, fluffy alveolar infiltrates with prominent air bronchograms ARDS Patchy abnormalities with increased density in dependent lung zones. • Barotrauma: Alveolar injury from exposure to excessive pressures • Volutrauma: Repetitive opening and closing of alveoli causing shear stress and triggering further inflammation • Atelectotrauma: During PPV, atelectatic regions will inflate, however the alveoli will be unstable and will collapse during the expiratory phase of the breath • Biotruama: lung suffering injury from any mediators of the inflammatory response or from bacteremia. INTUBATION AND PEEP • Worsening lung disease clinically and on radiographs • Hypoxemia despite facemask (provides 50% - 90% FiO2) ARDS • Increased work of breathing secondary to inadequate minute ventilation, increased dead space, decreased functional residual capacity (FRC), decreased lung compliance TO MINIMIZE LUNG INJURY NEJM 2000 ARDS NETWORK STUDY • Increase PEEP to recruit alveoli, increase mean airway pressure, improve oxygenation, increase functional residual capacity, and decrease alveolar edema • Use enough PEEP (range from 8 to 20) to allow for FiO2 <0.6 • higher FiO2 exposes the lung to oxygen toxicity • Lower oxygen saturations of 88%-95% or paO2 55-80mmHg can be accepted in order to minimize oxygen toxicity • If permissive hypoxia is being accepted, assure adequate end organ oxygen delivery TO MINIMIZE LUNG INJURY NEJM 2000 ARDS NETWORK STUDY • Low tidal volume with a goal of 6 ml/kg should be used • This goal tidal volume can be decreased if plateau airway pressures are > 30cm H2O. Therefore goal plateau pressure is <30 • The preferred mode of ventilation is time cycled, pressure regulated, and volume controlled • Set a ventilatory rate to achieve pH 7.3-7.45. Often, permissive hypercapnia with a pH down to 7.2 is allowed to minimize ventilatorinduced lung injury. • Be vigilant of I-time, inspiratory time, while setting the rate. A longer I-time optimizes pulmonary recruitment HIGH FREQUENCY VENTILATION (HFV) • Advantages • Use of low VT • Avoidance of barotrauma • Maintenance of near normal PaCO2 • Disadvantages • Patient must remain paralyzed FLUIDS • aggressively fluid resuscitate for any signs / symptoms of shock in order to optimize end organ perfusion • fluid administration should be minimized to decrease the alveolar-capillary leak and pulmonary edema • Aggressive diuresis should also be considered once hemodynamic stability is achieved • Nutritional support should be initiated as early as possible (TPN or enterally) PRONE • Improve regional lung perfusion and oxygenation • The greatest effects are achieved when patients are maintained prone for 12 hours or longer and oxygenation improves in 6070% of patients. NITRIC OXIDE • Short half-life, selective pulmonary vasodilator, no systemic hemodynamic effects • Improves oxygenation and V/Q matching by increasing blood flow to well ventilated lung and shunting blood away from poorly ventilated lung • Decreases pulmonary pressures thereby reducing right ventricular afterload • Initial improvements of oxygenation (~60% of patients) however no sustained improvement after 1-2 da • No reduction in morbidity/mortality • iNO may be used as a temporary rescue when there is refractory hypoxemia with conventional treatment CORTICOSTEROIDS • Anti-inflammatory actions of steroids have not been shown to be beneficial in the early course of ARDS. • Prospective adult study showed methyl-pred reduces lung injury in ARDS >7 days • May be helpful in fibrotic phase to help wean patients from the ventilator. Surfactant ECMO • Theoretical derangements in surfactant composition • Although shown to be beneficial in neonates with RDS, not recommended in ARDS • Lacking proof to any benefit • Reserved for those that do not respond to conventional management PROGNOSIS • • • Mortality in children and adults with ARDS are similar. Average 52% (range 28.5%-90%) Improved management of children with ARDS has decreased mortality rates (currently described as 30-50% Death is primarily due to sepsis or multiple organ dysfunction • Poor prognostic factors include • failure of oxygenation improvement after 6 days • high oxygenation index • liver dysfunction/cirrhosis • non-pulmonary organ dysfunction • organ transplantation • active malignancy • right ventricular dysfunction • PaO2/FiO2<100 • sepsis TAKE HOME POINTS ARDS is a process of diffuse pulmonary inflammation with increased vascular and alveolar permeability • The physiologic process is restrictive lung disease and hypoxemia occurs due to edema and V/Q mismatch • The ventilatory treatment involves increasing mean airway pressure to improve oxygenation • Ventilator induced lung injury can be minimized by maintaining high PEEP, low tidal volume, peak pressures < 35 cm H2O, and FiO2 < 60% • Death is primarily due to multi-organ dysfunction (usually cardiovascular collapse), and not ARDS primarily RESOURCES • Patel, SR, Karmpaliotis, D, Ayas, NT, et al. The role of open-lung biopsy in ARDS. Chest 2004; 125:197. • Papazian, L, Thomas, P, Bregeon, F, et al. Open-lung biopsy in patients with acute respiratory distress syndrome. Anesthesiology 1998; 88:935. • Rubenfeld, GD, Caldwell, E, Peabody, E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353:1685. • Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301.