Urine Proteomics in Kawasaki Disease
advertisement

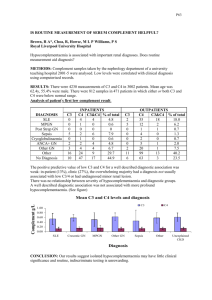
Urine Proteomics in Kawasaki Disease Pawan Sharma 15th October 2013 Kawasaki Disease • Uncommonly common systemic vasculitis. • 6 months to 4 years age. • Significant mortality and morbidity esp with delayed diagnosis. • No pathognomic test for early diagnosis. GOAL To discover and validate diagnostic markers of KD in a prospective cohort. . Study participants • Over 39 months in Tertiary care hospital. • Approved by Boston Children’s Hospital Committee. • Patients under the age of 18 with possible diagnosis of KD. • Exclusion criteria: neoplastic, renal or urologic disease. • Total patients mentioned 236 (234). Study design • Discovery phase. • Validation phase: First cohort based on possible KD, but before determination of final diagnosis. Second cohort utilized serum specimens collected as a part of Pediatric Heart Network Study of KD. Out come measures • Paediatric Rheumatologist • Use of Published Diagnostic Criteria for KD. • Atypical KD was established using American Heart association guideline. DF candidate diagnostic marker • Analysed 15 patients. • 6 KD patients (3 with and 3 without Coronary heart disease). • 6 non-KD patients (2 non specific, 3 adeno and 1 Pyelonephritis). • 3 matched specimen from treated patients with KD (after 1 month). • 190 proteins specific to KD. • Meprin A and Filamin C chosen. Urine proteomics for discovery of improved diagnostic markers of Kawasaki disease EMBO Molecular Medicine Volume 5, Issue 2, pages 210-220, 20 DEC 2012 DOI: 10.1002/emmm.201201494 http://onlinelibrary.wiley.com/doi/10.1002/emmm.201201494/full#fig1 Validation • • • • • Prospectively measured concentration in urine. Investigators blinded to Final diagnosis. Mean age 3 years. 53 (49%) final diagnosis of KD. All treated with IVIg and Aspirin, 30% required repeat treatment. • All studied patients received a Final Outcome. Table 2. Final diagnosis of the 107 study patients Final diagnosis Number of patients Kawasaki disease 53 (49%) Viral syndrome 33 (61%) Adenovirus 6 Serum sickness 3 Pyelonephritis 2 group A streptococcal pharyngitis 2 Cytomegalovirus 1 Epstein-Barr virus 1 Group A streptococcal pharyngitis 1 Lyme disease 1 Otitis media 1 Pneumonia 1 Respiratory syncytial virus 1 Systemic arthritis 1 Mean values KD Non KD Filamin C 19.2 3.7 Meprin A 50.2 5.6 Atypical KD Non KD Filamin C 17 3.7 Meprin A 41.5 5.6 Blinded case control study Response to treatment 2nd Cohort • 112 archived samples collected from KD patient analysed. • Compared them with Non-KD febrile illness. • Serum samples used. • Results were: KD Non KD Filamin C 217 6.6 Meprin A 1363 14.8 Mouse model What do we think • Clear question for study address? • Was there a comparison with appropriate standard? • Did all patients get diagnostic test and reference standard? • Could the result have been influenced? • Is disease status clearly described? • Were methods described in clear details? • What are the results? • Can the results be applied to our patients? Summary • Potential approaches for improving diagnosis. • Discover phase: very small group. • Mechanism of how this markers accumulate shall be important direction for future work. • Limitations: Renal or Urologic disease, sever dehydration or ? Shock. • Thank you! The Receiver Operating Characteristic Curve. • true positive rate (Sensitivity) is plotted in function of the false positive rate (100-Specificity) for different cut-off points. • Each point on the ROC curve represents a sensitivity/specificity pair corresponding to a particular decision threshold. • A test with perfect discrimination has a ROC curve that passes through the upper left corner (100% sensitivity, 100% specificity). Therefore the closer the ROC curve is to the upper left corner, the higher the overall accuracy of the test . ROC Chart