Core Evidence - Yorkshire and the Humber Deanery
advertisement

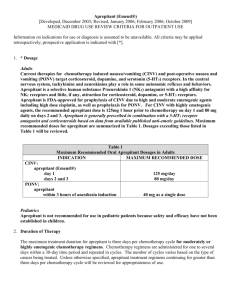
Fosaprepitant and aprepitant Dr Adam Hurlow 16/11/11 Fosaprepitant and aprepitant • Selective neurokinin-1 receptor antagonists • Aprepitant PO/fosaprepitant IV prodrug • Fosaprepitant 115mg equivalent to aprepitant 115mg • Licensed for treatment of chemotherapy induced nausea and vomiting (CINV) with highly and moderately emetogenic chemotherapy Substance P/NK1R • Substance P –tachykinin • Acts on NK1 receptor • Found in the area postrema (CTZ), nucleus tractus solitarri (NTS), vomiting centre • Exogenous Substance P in NTS triggers vomiting • Substance P/NK1R within the final common pathway to regulate vomiting Pathophysiology of Chemotherapy-Induced Emesis CINV • Acute (post-treatment) – Occurs within first 24 hours after administration of cancer chemotherapy • Delayed – – CINV that begins after first 24 hours May last for 120 hours • Anticipatory – Learned or conditioned response from poorly controlled nausea and vomiting associated with previous chemotherapy • Breakthrough – CINV that occurs despite prophylaxis and requires rescue • Refractory – Occurs during subsequent treatment cycles when prophylaxis and/or rescue has failed in previous cycles CINV • 50% of patients receiving high-dose cisplatin experienced vomiting and 58% experienced nausea despite standard therapy • Anthracycline and cyclophosphamide chemotherapy for breast cancer evoked vomiting in 41% of patients and nausea in 67% following ondansetron and dexamethasone Perception vs. Reality: Emetogenic Chemotherapy Highly Emetogenic Chemotherapy Grunberg S. Cancer. 2004;100:2261-2268. Moderately Emetogenic Chemotherapy Fosaprepitant and aprepitant in CINV • Recommended in following guidelines fro highly/moderately emetogenic chemotherapy: • American society of clinical oncology, 2006 • European Society of medical oncology, 2008 • Multinational association of supportive care in cancer,2008 • National comprehensive cancer network,2008 Emetogenic Potential of Single Antineoplastic Agents HIGH Risk in nearly all patients (> 90%) MODERATE Risk in 30% to 90% of patients LOW Risk in 10% to 30% of patients MINIMAL Fewer than 10% at risk Evidence base • Cochrane protocol but no review • Recent literature reviews Chrisp P Core Evidence 2007;2(1) & Langford P and Chrisp P Core Evidence 2010:5 77-90 • 2007 - 1 meta-analysis, 13 RCT, 1 case reports • 2010 – 1 meta analysis, 4 RCT, 2 case reports • Both concluded – clear evidence adding aprepitant to dexamethasone plus a serotonin antagonist improves control of emesis and nausea and reduces need for rescue medication in patients receiving moderately or highly emetogenic chemotherapy • Clear evidence patients more satisfied with their antiemetic therapy when aprepitant added; less impact of symptoms on daily activities Evidence: Aprepitant & standard therapy (ST) vs. ST and placebo Acute complete response % Delayed complete response % Navari 1999 77 vs. 57 (p =0.004) 52 vs. 16 (p <0.001) Campos 2001 75 vs. 51 (p<0.01) 41 vs. 22 (p<0.05) Hesketh 2003 89.2 vs. 78.1 (<0.001) 75.4 vs. 55.8 (<0.001) Poli-bigelli 2003 82.8 vs. 68.4 (<0.001) 67.7 vs. 46.8 (<0.001) Gralla 2005 71 vs. 49 (<0.005) 67 vs. 32 (<0.005) Warr 2005 86 vs. 73 (<0.001) 72 vs 51(<0.001) Schmoll 2006 87.7 vs 79.3 (<0.005) 74.1 vs 63.1 (<0.004) Herrington 2008 66.7 -70.4 vs 56.2 63-59.3 vs 31.2 Yeo 2009 72.1 vs 72.6 75.6 vs 67.4 Aprepitant beyond chemo • Preventing postoperative nausea and vomiting: post hoc analysis of pooled data from two randomized active-controlled trials of aprepitant. Current Medical Research and Opinion2007, Vol. 23, No. 10 , Pages 2559-2565 Diemumsch P et al - 1599 patients for major surgery under general anaesthesia - RCT, double blind - aprepitant 40mg or125mg vs ondansetron 4mg IV pre-op - no significant nausea (56.4% vs. 48.1%) - no nausea (39.6% vs. 33.1%) - no vomiting (86.7% vs. 72.4%) - no nausea and no vomiting (38.3% vs. 31.4%) - no nausea, no vomiting, and no use of rescue (37.9% vs. 31.2%) p < 0.035 for the odds ratio for each comparison Regimens • Fosaprepitant (Ivemend) intravenous infusion, over 20–30 minutes, 150 mg 30 minutes before chemotherapy on day 1 of cycle only • Aprepitant (Emend) 125 mg 1 h pre chemotherapy, then 80 mg od for the next 2 days Complications • Aprepitant is eliminated primarily by metabolism; aprepitant is not renally excreted. • Well tolerated in patients with mild to moderate hepatic insufficiency (Child-Pugh 59). Unknown >9 • No dose adjustment for renal insufficient/HD • Side effects diarrhoea (23-60%), headache 3%, infusion site pain 7.6-10.4% Interactions • CYP3A4 substrate - increased by ketocoanzole - decreased by carbemazapine • inhibition of CYP3A4 and induction CYP2C9 - increases dex/methylpred levels - OCP failure - increases midazolam - decreases warfarin