ASSENT 3 Slides - Clinical Trial Results
advertisement

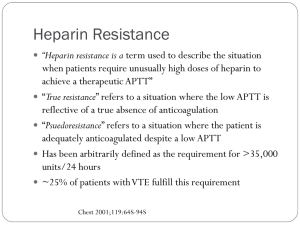
ASSENT 3 Efficacy and Safety of Tenecteplase in Combination with Enoxaparin, Abciximab or Unfractionated Heparin: the ASSENT-3 Randomised Trial in Acute Myocardial Infarction The Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 Investigators The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT-3 Trial Design ST-Segment Elevation AMI (6095 patients) 150-325 mg Aspirin (daily) Randomized Full-Dose TNK-tPA Plus Enoxaparin Half-Dose TNK-tPA Plus Abciximab Plus Low-Dose Heparin Full-Dose TNK-tPA Plus Weight-Adjusted Heparin The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Rationale for Inclusion of TNK-tPA Monotherapy and Unfractionated Heparin • TNK-tPA plus UFH (fully weight-adjusted) – In spite of several 100,000s of patients studied in thrombolysis trials, the optimal dose of concomitant UFH remains unknown – InTIME-II data with t-PA suggest lower ICH rates with reduced, fully weight-adjusted UFH, and earlier aPTT monitoring – AHA/ACC-recommended fully weight-adjusted dose of UFH never tested prospectively in a large-scale trial The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. Pathophysiology of Combination Therapy in AMI Combination Therapy Thrombus Reduces Reinfarction* % Stenosis Myocardial Blush Minimum Diameter ST Resolution Epicardial Flow Facilitates PCI Myocardial Flow *Gibson et al. J Am Coll Cardiol. 1995;25:582-589. Gibson et al. Circulation. 2001;103:2550-2554. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. Angiographic Predictors of Reocclusion Incidence of reocclusion (%) P=0.003 12 P=0.03 20 10.4 P=0.009 12 18.2 10 10 16 14 8 9 10.7 18 6 2 5.6 6 4 4 3.0 2 2 0 TIMI 2 TIMI 3 TIMI Flow 0 8 78 7 77 77.9 76 + – Collaterals 0 75 4 8 2.2 79 8.4 5 10 4 P=0.04 6 8 12 6 P=0.06 + Ulcer – 3.3 3 2 73 1 72 0 + – Thrombus 73.9 74 71 + – % Stenosis Gibson et al. J Am Coll Cardiol. 1995;25:582-589. Clinical Impact of Reocclusion: 810 patients, cath 90 min & 7 days later: 12.4% reocclude, 58% symptomatic, in-hospital mortality 11.0% vs 4.5% (P=0.01). Ohman et al. Circulation. 1990;82:781-791. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. Thrombus Remains Following Thrombolysis: The Need For Aggressive Antithrombotic / Antiplatelet Therapy When a small camera is placed in the artery (angioscopy), all 40 thrombolytic patients in a study by Van Belle et al had some remaining form of thrombus (shown in red here). While thrombolysis reduces thrombus burden, thrombus remains, & thrombolysis exposes underlying ulcerated lesions. The high frequency of persistent thrombotic lesions underscores the need for effective antithrombotic therapy following thrombolytic administration. Van Belle et al. Circulation. 1998;97:26-33. ASSENT 3: Rationale for Use of Enoxaparin • TNK-tPA plus enoxaparin – Favorable effects of LMWHs in recent small-scale thrombolysis trials – Higher late patency: HART-2 ASSENT-Plus AMI-SK – Less reocclusion: HART-2 – Fewer reinfarctions: ASSENT-Plus AMI-SK Wilson et al • ASSENT 3 is the first large-scale trial to test LMWH The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Rationale for Use of Enoxaparin • Enoxaparin Advantages – Reduced bleeding (because it binds less strongly to circulating plasma, proteins, etc.) – Superior bioavailability at lower doses – Longer half-life – Does not require aPTT monitoring, due to more predictive dose response – Possible long-term cost advantage due to lower frequency of aPTT monitoring – Delivered subcutaneously with potential for even longer durations of administration – Enoxaparin has unique anti-Xa:anti-IIa ratio that distinguishes it from others in its class The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Rationale for Half Dose TNK-tPA Plus FullDose Abciximab Trial arm based on hypothesis that a thrombolytic agent plus GP llb/llla inhibitor might result in improved patency with enhanced safety • Hypothesis based on – Phase II angiographic trials that suggest there may be improved patency with half-dose lytic plus GP llb/llla inhibitor – Enhanced safety profile of TNK-tPA, reduced risk of major complications, especially in the elderly (ASSENT 2) The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Inclusion Criteria • Inclusion criteria were identical to those of the Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-2 trial: Age 18 years or older Onset of symptoms within 6 hours before randomization ST-segment elevation of 1 mm or more in two or more limb leads, or 2 mm or more in two or more contiguous precordial leads or left bundle-branch block. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Exclusion Criteria Exclusion criteria on admission were: • Systolic blood pressure of more than 180 mm Hg • Diastolic blood pressure of more than 110 mm Hg, or both on repeated measurements • Use of abciximab or other glycoprotein IIb/IIIa inhibitors within the preceding seven days • Major surgery, biopsy of a parenchymal organ or substantial trauma within two months • Any head or other trauma occurring after onset of current myocardial infarction; any known history of stroke, transient ischemic attack or dementia; any known structural damage to the central nervous system • Current therapy with oral anticoagulants; treatment with unfractionated heparin 5,000 U or a therapeutic subcutaneous dose of low-molecular-weight heparin within 6 hours The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Exclusion Criteria (continued) Exclusion criteria on admission were (continued): • Known thrombocytopenia (100,000 cells/l) • Known renal insufficiency (serum creatinine 2.5 mg% for men and 2.0 mg% for women) • Sustained cardiopulmonary resuscitation (more than 10 min) in previous two weeks • Pregnancy, lactation, or parturition in the previous 30 days • Active participation in another investigative drug or device study in the previous 30 days; previous enrolment in this study • Inability to follow the protocol and to comply with the follow-up requirements The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: TNK-tPA Weight-Adjusted Dosing Weight (kg) Full dose (mg) Half dose (mg) (in combination with abcix) <60 (<132 lbs) 30.0 15.0 ≥60 to <70 (133-154 lbs) 35.0 17.5 ≥70 to <80 (155-176 lbs) 40.0 20.0 ≥80 to <90 (177-198 lbs) 45.0 22.5 ≥90 (>199 lbs) 50.0 25.0 The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Trial Design An international, multicenter, randomized (1:1:1), open-label, controlled, parallelgroup study in patients with ST-elevation AMI presenting within 6 hours of symptom onset, treated with 1 of 3 different reperfusion regimens randomization 1:1:1 enoxaparin IV bolus UFH IV bolus UFH IV bolus Wt adj TNK-tPA full-dose IV bolus abciximab IV bolus Wt adj TNK-tPA full-dose IV bolus enoxaparin SC injections every 12 hours up to discharge or revascularization (max of 7 days) Wt adj TNK-tPA half-dose IV bolus UFH IV infusion for up to 48 hours abciximab IV infusion for 12 hours UFH IV infusion for up to 48 hours The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Primary Endpoints Primary Efficacy Endpoint: Composite of 30-day mortality or in-hospital reinfarction or in-hospital refractory ischemia. Primary Efficacy Plus Safety Endpoint: Composite of 30-day mortality or in-hospital reinfarction or inhospital refractory ischemia plus in-hospital intracranial haemorrhage or in-hospital major bleeding other than intracranial. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Statistical Analysis Plan • Statistical analysis was by intention-to-treat. • A two-sided 95% confidence interval was calculated and an overall chi-square test, comparing the three treatment groups, was performed. • Between groups pair wise comparisons are presented using the twosided 95% confidence interval of the relative risk. • Non-parametric, covariate-adjusted rates were calculated for each end point. Covariates used were gender, age, weight, infarct location, previous infarct, Killip class, heart rate, time to tenecteplase treatment and systolic blood pressure. • Since the results of the adjusted and non-adjusted analyses were very similar only non-adjusted results are presented. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Statistical Power • The study had 80% power to exclude with 95% confidence (one-sided) a 1% higher incidence of these end points in comparison with the control group The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Endpoint Adjudication • Stroke: Reviewed blinded to treatment allocation by the same stroke committee that reviewed the stroke data in the ASSENT-2 trial. • Reinfarction, refractory ischemia, bleeding complications: No central adjudication. However, definitions were provided to the investigators who, in addition, had to reconfirm the occurrence of these end points on a special form. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Endpoint Definitions • Reinfarction in the first 18 hours: Recurrent symptoms of ischemia at rest accompanied by new or recurrent ST-segment elevations of 0.1 mV or more in at least two contiguous leads, lasting at least 30 min. • Reinfarction after 18 hours: New Q waves in two or more leads and/or enzyme evidence of reinfarction (re-elevations of CK-MB troponins or total CK above the upper limit of normal and increased over the previous value). • Refractory ischemia: Symptoms of ischemia with ST-segment deviation or T wave inversion persisting for at least 10 min despite medical management and not fulfilling the diagnosis of reinfarction. • Non-cerebral bleeding complications were defined as major (requiring transfusion, intervention because of hemodynamic compromise or both) or minor. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Randomization and Study Treatments 6095 patients with ST-segment elevation acute myocardial infarction 2040 assigned full-dose TNK-tPA plus enoxaparin 2017 assigned half-dose TNK-tPA plus weight-adjusted, reduced dose, unfractionated heparin plus abciximab At least 1 component of study medication not given: 21 Primary composite end points unavailable: 3 2037 completed follow-up 2038 assigned full-dose TNK-tPA plus weight-adjusted unfractionated heparin At least 1 component of study medication not given: 61 Primary composite end points unavailable: 1 2016 completed follow-up At least 1 component of study medication not given: 24 Primary composite end points unavailable: 2 2036 completed follow-up The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Concomitant Use of Medications In-Hospital Enoxaparin (n=2040) Abciximab (n=2017) Unfractionated Heparin (n=2038) Calcium channel blockers 11 10 11 0.82 IV nitrates 73 71 73 0.25 Beta-blockers 84 84 83 0.73 ACE inhibitors 62 60 63 0.18 Angiogenesis II inhibitors 3.1 2.9 3.1 0.87 Statins 52 50 51 0.24 Aspirin ≤12h or upon randomization in-hospital 97 96 97 95 97 95 0.53 0.40 Ticlopidine/Clopidogrel 30 28 32 0.014 Oral anticoagulants 4.3 4.8 5.2 0.41 Abciximab 6.5 4.2 8.9 <0.0001 Other GP IIb/IIIa inhibitors 6.8 3.7 7.1 <0.0001 Low-molecular-weight heparins 16.0 26.0 29.0 <0.0001 Thrombolytics 2.0 1.9 2.9 P Value 0.08 The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Baseline Characteristics Enoxaparin (n=2040) Abciximab (n=2017) Unfractionated Heparin (n=2038) Age (years) 61 61 61 0.41 Age >75 years 13 12 13 0.21 Women 23 24 23 0.40 Weight (kg) 79 79 79 0.73 Height (cm) 170 170 170 0.51 Time from onset of symptoms to randomization (h) 2.7 2.7 2.8 0.33 Time from randomization to TNK-tPA (h) 0.26 0.40 0.27 <0.0001 Killip class I II/III IV Systolic blood pressure (mm Hg) 89 10 0.3 88 11 0.4 88 12 0.4 134 133 133 3 way P Value 0.61 0.66 Data are mean (SD) or percentages. CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Baseline Characteristics (Cont.) Enoxaparin (n=2040) Abciximab (n=2017) Unfractionated Heparin (n=2038) P Value Infarct location Anterior Inferior Other 0.91 39 56 4.6 39 56 4.8 38 57 5.0 Heart rate (bpm) 75 75 74 0.23 Hypertension 41 41 41 0.99 Diabetes 19 18 18 0.66 Previous myocardial infarction 14 13 14 0.52 Prior CABG 3.6 3.3 2.6 0.19 Prior PCI 6.2 6.0 6.4 0.86 Current smoker 44 47 47 0.16 Data are mean (SD) or percentages. CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. Baseline Demographics of Large Scale Thrombolytic Trials GUSTO I 90-93 Patients Randomized (ITT) 30647 GUSTO III 95-97 15059 InTIME II 97-99 ASSENT II 97-98 GUSTO V 99-01 ASSENT 3 00-01 ASSENT 3 ASSENT 3 00-01 00-01 15060 16949 16588 2040 2017 2038 Female, (%) 25 27 25 23 25 23 24 23 Age (median years) 62 63 62 61 61 61 61 61 Previous MI (%) 17 18 16 16 15 14 13 14 Diabetes (%) 15 16 14 16 16 19 18 18 Anterior MI (%) 39 48 42 40 37 39 39 38 Median Time (hrs) Between Symptom and First Study Rx 2.8 2.7 2.9 2.8 2.7 2.7 2.7 2.8 The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Primary Composite Endpoints at Hospital Discharge and at 30 Days Enoxaparin (n=2040) Abciximab (n=2017) Unfractionated Heparin (n=2038) 3 way P Value 30-day mortality or in-hospital reinfarction or in-hospital refractory ischemia 11.4 11.1 15.4 <0.0001 30-day mortality or in-hospital reinfarction or in-hospital refractory ischemia or in-hospital ICH or in-hospital major bleeds (other than ICH) 13.8 14.2 17.0 0.0081 Data are percentages and 95% confidence intervals. ICH, intracranial hemorrhage. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Days to Death or Reinfarction or Refractory Ischemia 20 Unfractionated Heparin 18 Probability (%) 16 14 15.4% Enoxaparin 12 11.4% 11.1% Abciximab 10 8 6 4 Log-rank test: P=0.0001 2 0 0 5 10 15 20 25 30 Days to Death or Reinfarction or Refractory Ischemia The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Days to Death or Reinfarction or Refractory Ischemia or ICH or Major Bleeding 20 Unfractionated Heparin 18 Probability (%) 16 17.0% Abciximab 14 14.2% 13.8% Enoxaparin 12 10 8 6 4 Log-rank test: P=0.0062 2 0 0 5 10 15 20 25 30 Days to Death or Reinfarction or Refractory Ischemia or ICH or Major Bleeding The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: 30 Day Mortality, Recurrent MI, Refractory Ischemia % Risk of 30 Day D / MI / Ref Isch 3 way P=0.0001 20.0% p=0.0009* p=0.0002* 15.4% 15.0% 11.4% 11.1% TNK + Enoxaparin TNK + Abciximab 10.0% 5.0% 0.0% TNK *P values are the Bonferroni p-values after correcting for multiple comparisons. The uncorrected p-values were p=0.0002 for the enox vs UFH comparison, and <0.0001 for the abcix vs UFH comparison. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. % Risk of 30 Day D / MI / Ref Isch / Maj Bleed / ICH ASSENT 3: 30 Day Mortality, Recurrent MI, Refractory Ischemia, Bleeding, ICH RefractoryMajor Ischemia 3 way p=0.0062 p=0.0146* p=0.057* 20.0% 17.0% 15.0% 13.8% 14.2% TNK + Enoxaparin TNK + Abciximab 10.0% 5.0% 0.0% TNK *P values are the Bonferroni p-values after correcting for multiple comparisons. The uncorrected p-values were p=0.0037 for the enox vs UFH comparison, and 0.0142 for the abcix vs UFH comparison. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Odds Ratios for Death at 30 Days or InHospital Reinfarction or Refractory Ischemia Death at 30 Days or In-Hospital Reinfarction or Refractory Ischemia (%) UFH Overall event rate Gender Male Female Infarct location Anterior Other Time to TNK-tPA (h) 0-2 ENOX or ABCIX Relative Risk (95% CI) 15.4 11.4 11.1 0.74 (0.63, 0.87) 0.72 (0.61, 0.84) 14.8 10.4 9.8 15.1 14.8 0.70 0.66 0.87 0.85 (0.58, (0.55, (0.65, (0.64, 0.84) 0.80) 1.17) 1.14) 14.6 13.6 9.5 9.5 0.75 0.70 0.72 0.73 (0.60, (0.56, (0.58, (0.58, 0.94) 0.88) 0.91) 0.91) 13.0 8.9 9.8 10.9 12.6 13.3 0.77 0.53 0.69 0.76 0.77 0.82 (0.59, (0.38, (0.53, (0.60, (0.56, (0.60, 1.01) 0.74) 0.84) 0.96) 1.06) 1.10) 17.4 19.4 13.0 16.8 >2-4 14.2 >4 16.3 ENOX vs UFH ABCIX vs UFH UFH, unfractionated heparin; ENOX, enoxaparin; ABCIX, abciximab. ENOX or ABCIX Better 0.5 UFH Better 1 The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. 2 ASSENT 3: Odds Ratio for Death at 30 Days or InHospital Reinfarction or Refractory Ischemia (Cont.) Death at 30 Days or In-Hospital Reinfarction or Refractory Ischemia (%) UFH Age (years) ≤75 13.8 >75 26.2 Diabetes Yes 13.8 No 15.8 ENOX or ABCIX Relative Risk (95% CI) 10.0 9.0 20.9 26.6 0.73 0.65 0.80 1.02 (0.61, (0.54, (0.59, (0.76, 0.87) 0.78) 1.09) 1.36) 12.4 18.0 11.2 9.5 0.90 1.31 0.71 0.60 (0.62, (0.93, (0.60, (0.50, 1.30) 1.84) 0.85) 0.72) ENOX vs UFH ABCIX vs UFH UFH, unfractionated heparin; ENOX, enoxaparin; ABCIX, abciximab. ENOX or ABCIX Better UFH Better * 0.5 1 * There was a statistically significant interaction between treatment with abciximab and diabetes, such that diabetics had poorer outcomes with abciximab therapy (p=0.0004). The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. 2 ASSENT 3: Odds Ratios for Days to Death or Reinfarction or Refractory Ischemia or ICH or Major Bleeding Death at 30 Days or In-Hospital Reinfarction or Refractory Ischemia or ICH or Major Bleeding (%) UFH Overall event rate Gender Male Female Infarct location Anterior Other Time to TNK-tPA (h) 0-2 ENOX or ABCIX Relative Risk (95% CI) 17.0 13.7 14.2 0.81 (0.70, 0.93) 0.84 (0.73, 0.97) 16.2 11.9 12.55 20.1 20.0 0.73 0.77 1.01 1.01 (0.61, (0.65, (0.78, (0.78, 0.88) 0.92) 1.31) 1.30) 16.0 16.6 12.3 12.8 0.78 0.83 0.82 0.85 (0.63, (0.67, (0.67, (0.70, 0.96) 1.02) 1.00) 1.03) 16.2 13.0 11.9 13.5 13.9 16.9 0.88 0.71 0.75 0.87 0.77 0.94 (0.69, (0.53, (0.60, (0.70, (0.57, (0.71, 1.14) 0.95) 0.94) 1.07) 1.05) 1.23) 19.9 20.5 15.0 18.3 >2-4 15.8 >4 18.0 ENOX vs UFH ABCIX vs UFH UFH, unfractionated heparin; ENOX, enoxaparin; ABCIX, abciximab. ENOX or ABCIX Better 0.5 UFH Better 1 The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. 2 ASSENT 3: Odds Ratio for Death, Reinfarction or Refractory Ischemia, ICH or Major Bleeding (Cont.) Death at 30 Days or In-Hospital Reinfarction or Refractory Ischemia or ICH or Major Bleeding (%) UFH Age (years) ≤75 15.4 >75 28.0 Diabetes Yes 16.5 No 17.2 ENOX or ABCIX Relative Risk (95% CI) 12.0 11.2 25.5 36.9 0.78 0.74 0.91 1.30 (0.66, (0.63, (0.69, (1.01, 0.92) 0.88) 1.20) 1.68) 13.9 22.3 13.7 12.5 0.84 1.35 0.80 0.74 (0.60, (1.00, (0.68, (0.62, 1.19) 1.82) 0.94) 0.87) ENOX vs UFH ABCIX vs UFH UFH, unfractionated heparin; ENOX, enoxaparin; ABCIX, abciximab. ENOX or ABCIX Better 0.5 UFH Better 1 *There was a statistically significant interaction between treatment with abciximab and diabetes, such that diabetics had poorer outcomes with abciximab therapy (p=0.0007), and likewise, patients over the age of 75 had poorer outcomes with abciximab (p=0.0010). The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. 2 % Risk of 30 Day Efficacy & Safety Endpoint ASSENT 3:ASSENT Primary Efficacy and Safety Endpoint of Death, 3: 30 Day Mortality Reinfarction or Refractory Ischemia, ICH or Major Bleeding in Patients > 75 Years of Age *p=0.001 40.0% 36.9% 35.0% 30.0% 28.0% 25.5% 25.0% 20.0% 15.0% 10.0% 5.0% 0.0% TNK + Enoxaparin TNK + Abciximab TNK *There was a statistically significant interaction between treatment with abciximab and age such that patients over the age of 75 had poorer outcomes with abciximab (p=0.0010). The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. % Risk of 30 Day Efficacy & Safety Endpoint ASSENT 3: Primary Efficacy and Safety Endpoint of Death, Reinfarction or Refractory Ischemia, ICH or Major Bleeding in Patients with Diabetes *p=0.0007 25.0% 22.3% 20.0% 16.5% 15.0% 13.9% 10.0% 5.0% 0.0% TNK + Enoxaparin TNK + Abciximab TNK *There was a statistically significant interaction between treatment with abciximab and diabetes, such that diabetics had poorer outcomes with abciximab therapy (p=0.0007). The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Frequency of Individual Endpoints at Hospital Discharge and at 30 Days Enoxaparin (n=2040) Abciximab (n=2017) Unfractionated Heparin (n=2038) 3 way P Value Death at 30 days 5.4 6.6 6.0 0.25 In-hospital reinfarction 2.7 2.2 4.2 0.0009 In-hospital refractory ischemia 4.6 3.2 6.5 <0.0001 In-hospital ICH 0.9 0.9 0.9 0.98 Major bleeding (other than ICH) 3.0 4.3 2.2 0.0005 Data are percentages. ICH, intracranial hemorrhage. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. % Risk of 30 Day Mortality ASSENT3: 3: 30 30 Day ASSENT DayMortality Mortality 3 way p=0.25 10.0% 6.6% 5.4% 6.0% 5.0% 0.0% TNK + Enoxaparin TNK + Abciximab TNK The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT DayDeath Mortality ASSENT 3: 3: 3030Day or MI % Risk of 30 Day Death or MI 3 way p=0.0198 10.0% 9.1% 6.8% 7.3% 5.0% 0.0% TNK + Enoxaparin TNK + Abciximab TNK The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. % Risk of In-Hospital Recurrent MI ASSENT 3: 30 Day Mortality ASSENT 3: In-Hospital Recurrent MI 3 way p=0.0009 5.0% 4.2% 2.7% 2.2% 0.0% TNK + Enoxaparin TNK + Abciximab TNK The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. % Risk of 30 Day Refractory Ischemia 3: 30 Day Mortality Ischemia ASSENTASSENT 3: In-Hospital Refractory 10.0% 3 way p< 0.0001 6.5% 5.0% 4.6% 3.2% 0.0% TNK + Enoxaparin TNK + Abciximab TNK The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. % Risk of Intracranial Hemorrhage ASSENT 3: 30 Day Mortality ASSENT In-Hospital ICH p=NS 5.0% 0.9% 0.9% 0.9% 0.0% TNK + Enoxaparin TNK + Abciximab TNK The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: 30ofDay Mortality ASSENT 3: Risk Major Bleeding 3 Way p = 0.005 % Risk of Major Hemorrhage p=NS p=0.0002 5.0% 4.3% 3.0% 2.2% 0.0% TNK + Enoxaparin TNK + Abciximab TNK The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Incidence of In-Hospital Thrombocytopenia and Noncerebral Bleeding Complications Any thrombocytopenia Thrombocytopenia <20,000 cells/µL 20,000 to 50,000 cells/µL 50,000 to <100,000 cells/µL Bleeding episodes Total Major Minor Blood transfusion Enoxaparin (n=2040) Abciximab (n=2017) Unfractionated Heparin (n=2038) 1.2 3.2 1.3 P Value 3 way <0.0001 <0.0001 0.1 0.2 0.9 * * * 3.4 * 25.6 3.0 22.6 0.5 0.6 2.0 0.2 0.2 1.0 39.7 4.3 35.4 21.1 2.2 18.8 <0.0001 0.0005 <0.0001 4.2 2.3 0.0032 * While 3 way p value is significant, Enoxaparin vs UFH comparison p=NS The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. % Risk of Major Hemorrhage ASSENT 3:ASSENT Risk of3:Major Bleeding in Patients 30 Day Mortality Over 75 Years 15.0% 13.3% 10.0% 4.1% 5.0% 0.0% TNK + Abciximab TNK The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. % Risk of Major Hemorrhage ASSENT 3:ASSENT Risk of3:Major Bleeding in Patients 30 Day Mortality With Diabetes 10.0% 7.0% 5.0% 2.2% 0.0% TNK + Abciximab TNK The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Frequency of In-Hospital Cardiac Events and Procedures Enoxaparin (n=2040) Abciximab (n=2017) Unfractionated Heparin (n=2038) P Value Sustained hypotension 2.1 2.8 2.8 0.27 Pulmonary edema and/or cardiogenic shock 5.2 5.2 5.6 0.78 Major arrhythmias 8.5 9.3 10.5 0.11 32.5 2.6 1.7 2.4 11.9 17.4 32.1 1.5 1.5 2.9 9.1 19.4 35.3 2.8 1.7 3.5 14.4 16.5 0.06 0.01 0.94 0.10 <0.0001 0.04 Invasive cardiac procedures Any IABP Urgent CABG Non-urgent CABG Urgent PCI Non-urgent PCI IABP, intra-aortic balloon pump; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Frequency of In-Hospital Cardiac Events and Procedures (Cont.) Enoxaparin (n=2040) Abciximab (n=2017) Unfractionated Heparin (n=2038) Pericarditis 0.7 1.0 0.6 0.23 Acute mitral regurgitation 0.1 0.3 0.3 0.35 Pulmonary embolism 0.1 0.3 0.1 0.19 Tamponade 0.4 0.3 0.3 0.89 Acute ventricular septum defect 0.5 0.4 0.5 0.85 Electromechanical dissociation 1.3 1.5 1.4 0.84 Killip class >1 4.5 4.9 4.8 0.82 0 0 0.1 0.67 Anaphylaxis P Value IABP, intra-aortic balloon pump; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: In-Hospital Stroke Rates Enoxaparin (n=2040) Abciximab (n=2017) Unfractionated Heparin (n=2038) Total strokes 1.62 1.49 1.52 0.94 Intracranial hemorrhage 0.88 0.94 0.93 0.98 Ischemic stroke* 0.64 0.40 0.54 0.57 Hemorrhagic conversion 0.07 0.07 0.00 0.77 Unclassified 0.15 0.15 0.05 0.59 P Value *Including hemorrhagic conversion The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Study Group Conclusions Regarding TNK + Abciximab Therapy • “The results obtained with half-dose tenecteplase plus abciximab are very similar to those with half-dose reteplase and abciximab seen in GUSTO-V.” • “In both trials, these benefits are obtained at the cost of a higher rate of major bleeding complications and blood transfusions”. • “No benefit and perhaps even harm was observed in patients above 75 years and in diabetics”. • “Taken together they suggest that caution should be exercised regarding the use of conjunctive therapy with abciximab in elderly patients with an acute myocardial infarction treated with a fibrinolytic agent.” The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Conclusions Regarding Enoxaparin • “In view of the present data and the ease of administration, enoxaparin might be considered an attractive alternative anticoagulant treatment when given in combination with tenecteplase”. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13. ASSENT 3: Unanswered Questions • “Whether enoxaparin is a desirable anticoagulant in conjunction with less fibrin-specific agents or whether enoxaparin can replace unfractionated heparin in combination with a platelet glycoprotein IIb/IIIa inhibitor and what role various pharmacologic combinations will ultimately have in conjunction with early coronary intervention needs to be determined”. The ASSENT 3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT 3 randomised trial. Lancet 2001;358:605-13.