IFN
advertisement
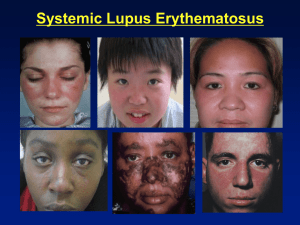
Systemic Lupus Erythematosus Emilio B. González, MD Professor and Director, Rheumatology UTMB May 18th, 2010 Systemic Lupus Erythematosus A chronic inflammatory systemic autoimmune disease of unknown etiology characterized by polyclonal Bcell activation and abnormal autoantibodies SLE – Epidemiology and Genetics Incidence: 1 in 1,000 -10,000 Female to male ratio: 9-1 More common in African-Americans but it affects all races Mean age of onset: 28 years Positive family history in 10 -15% of patients Monozygotic twins exhibit a greater rate of concordance (24%) than dizygotic twins (1-3%) Several complement deficiencies associated with SLE: C1q, C1r, C1s, C4, C2, C1 inhibitor deficiency, CR1 receptor deficiency Immunogenetics Increased Risk for SLE in: HLA-DR2 (anti-DNA Abs) HLA-DR3 (anti-Ro Abs) Null alleles at C2 and C4 loci SLE may be transmitted in an autosomal dominant pattern (family studies) SLE – Genetic Susceptibility MHC Related Not MHC Related HLA-DR1, 2, 3, 4 C1q deficiency (rare but highest risk) Alleles of HLA-DRB1, IRF5, Chromosome 1 region 1q41-43 and STAT4 C2 - C4 deficiency TNF- polymorphisms (PARP), region 1q23 (FcγRIIA, FcγRIIIA) IL-10, IL-6 and MBL polymorphisms Chromosome 8.p23.1: reduced expression of BLK and increased expression of C8orf13 (B cell tyrosine kinase), chromosome 16p11.22: integrin genes IGAM-ITGAX B cell gene BANK1 X chromosome-linked gene IRAK1 1982 ACR (Revised 1997) SLE Classification Criteria 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Malar (butterfly) rash Discoid lesions Photosensitivity Oral ulcers Non-deforming arthritis (non-erosive for the most part) Serositis: pleuropericarditis, aseptic peritonitis Renal: persistent proteinuria › 0.5 g/d or ›3+ or cellular casts Neurologic disorders: seizures, psychosis Heme: hemolytic anemia; leukopenia, thrombocytopenia Immune: anti-DNA, or anti-Sm, or APS (ACA IgG, IgM), or lupus anticoagulant (standard) or false + RPR Positive FANA (fluorescent antinuclear antibody) Definite SLE = 4 or more positive criteria SLE-Clinical and Laboratory Features Musculoskeletal Skin Renal CNS Severe thrombocytopenia Positive ANA 90% 80% 50% 15% 5-10% 95+% Also, cardiopulmonary involvement, thrombotic tendency (APS), and “premature” or accelerated atherosclerosis! Joint involvement in lupus mimics rheumatoid arthritis (RA) but milder Jaccoud’s arthropathy Arthritis in lupus can be deforming but is typically non-erosive! Autoantibodies Anti-dsDNA Lupus (occasionally other CTDs) ENA (anti-Sm and anti-RNP) SLE - MCTD - UCTD Anti-Ro and anti-La Sjögren’s, SLE, neonatal lupus Anti-Jo1 Polymyositis-Dermatomyositis Scl-70 Scleroderma Anti-centromere CREST Sx Anti-histone SLE and drug-induced lupus ENA = Extractable Nuclear Antigens Anti-Smith or anti-Sm: Anti-RNP (ribonucleoprotein): Almost exclusively seen in lupus but present only in about 30 percent of cases. Occasionally seen in other CTDs, e.g., MCTD High titers typically in MCTD but (+) also in lupus, PM-DM, scleroderma, Sjögren’s, UCTD, etc SLE – Pathogenetic Mechanisms Immune complex-mediated damage: glomerulonephritis Direct autoantibody-induced damage: thrombocytopenia and hemolytic anemia Antiphospholipid antibody-induced thrombosis Complement-mediated inflammation: CNS lupus (C3a), hypoxemia, and also anti-phospholipid mediated fetal loss Either failure of or abnormal response to normal apoptosis Anti-native DNA Fairly specific for SLE but present only in 60% of cases at best Titers correlate with disease activity Higher titers with nephritis DR2 gene association Can be useful for: Diagnosis Prognosis Therapeutic monitoring Immune-complex Injury in SLE DNA + Anti-DNA = DNA - Anti-DNA complex C3 C4 Tissue Injury SLE: Anti-DNA, C3, C4 Lupus – Complement Levels Patients who are always hypocomplementemic regardless of clinical disease activity may have an underlying complement deficiency! SLE – Pathogenesis The Dendritic cell – Alpha Interferon Hypothesis SLE – The Role of Dendritic Cells (DC) and Alpha Interferon (IFN ) Normally, resting DC mediate tolerance, i.e., no immune response to own tissues: they capture dead cells debris, and the immune system never encounters this waste DC become activated by viral infections, producing interferon. After viral infections resolve, interferon disappears DC proliferate and become activated when blood cells from normal donors are cultured with sera from lupus patients IFN identified as the primary substance responsible for this effect Pascual V, Banchereau J, Palucka KA. The central role of dendritic cells and interferon-alpha in SLE. Curr Opin Rheumatol. 2003; 15(5):548–556. SLE – The Role of Dendritic Cells (DC) and Alpha Interferon In lupus, the normal immune response appears altered as plasmacytoid dendritic cells (pDC) become hyperactivated by IFN Immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG induce IFN production in pDC Abnormal secretion of alpha interferon in lupus: the signature cytokine for the disease Dendritic cells activate B and T cells, leading to a chronic autoimmune state = lupus Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum 2004; 50 (6):1861-72 Cytokines in Systemic Lupus Erythematosus (SLE) and Rheumatoid Arthritis (RA) Many pro-inflammatory mediators, chemokines, and cytokines are involved in both diseases, however: In RA, mainly TNF In SLE, it appears that alpha interferon is the main pro-inflammatory cytokine Pascual V, Banchereau J, Palucka KA. The central role of dendritic cells and interferon-alpha in SLE. Curr Opin Rheumatol. 2003; 15(5):548–556. Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum 2004; 50 (6):1861-72 SLE – Cardiac Disease Pericarditis Inflammatory fluid Rarely tamponade Myocarditis Coronary vasculitis – Rare Libmann-Sachs endocarditis Premature or accelerated atherosclerotic disease Coronary Heart Disease in Lupus The prevalence ranges from 6 to 15% The incidence of myocardial infarction is five times higher in lupus than in the general population The risk of adverse cardiovascular outcomes is by a factor of 7 to 17 in patients with lupus as compared with the Framingham cohort Young women (between ages 35 and 44) are significantly more likely (52-fold increased risk) to experience an MI if they have lupus Ward MM. Arthritis Rheum 1999; 42(2): 338-46 Manzi S et al. Am J Epidemiol 1997; 145: 408-15 Petri M, et al. Am J Med 1992; 93: 513-9 Sturfelt G, et al. Medicine (Baltimore) 1992; 71: 216-23 Esdaile JM, et al. Arthritis Rheum 2001; 44: 2331-7 Leading Causes of Death in SLE Active lupus Infection Cardiovascular disease SLE - Mortality Study Site: Patient #: Deaths: California¹ 408 144 Toronto² 665 124 Active lupus: 49 (34%) 20 (16%) 19 (15.5%) Infection: 32 (22%) 40 (32%) 25 (20.5 %) CV disease: 23 (16%) 19 (15.4%) 32 (26.2%) 1. Ward MM, et al. A&R 1995; 38: 1492-9 2. Abu-Shakra M, et al. J Rheum 1995; 22: 1259-64 3. Jacobsen S, et al. Scand J Rheumatol 1999; 28: 75-80 Denmark³ 513 122 Lung Disease in Lupus Pleural disease Most common pulmonary involvement Inflammatory and exudative Chylothorax rarely* Interstitial lung disease Acute hypoxemia with normal CXR – Improves with steroids Alveolar hemorrhage – Typically in the setting of APS *Morgan C, Gonzalez E. Chylothorax as a rare complication in systemic lupus erythematosus. Poster presentation at the ACP-ASIM Georgia Chapter meeting, May 3-5, 2002 Renal Disease in Lupus Nephrotic and nephritic syndromes Glomerulonephritis Mesangial (type II WHO classification) Focal proliferative (type III WHO classification) Diffuse proliferative (type IV WHO (classification) Membranous (type V WHO classification) Tubulo-interstitial disease Burnt-out or sclerosed kidneys In a patient with newly diagnoses lupus, even if mild clinically, e.g., skin and joints, always check a UA so as to not miss an active urine sediment! Renal immunofluorescence in lupus - The “full house” effect: multiple (+) immune reactants: IgG, IgM, C1q, C3, C4, etc SLE – Heme Manifestations Autoimmune hemolytic anemia (AHA) Autoimmune thrombocytopenia, ITP-like Leukopenia Pancytopenia Lymphopenia Anti-phospholipid antibodies – False positive RPRs (neg FTA) Lymphadenopathy Rarely, aplastic anemia (from anti-stem cell antibodies) CNS Lupus Seizures - Epilepsy Strokes with hemiparesis Coma (“lupus cerebritis”) Cranial nerve and peripheral neuropathies Brain stem/cord lesions Aseptic meningitis Transverse myelitis Psychiatric: memory loss, cognitive changes Myasthenia gravis, multiple-sclerosis like Ro (SSA) and La (SSB) Primary Sjögren's Syndrome Neonatal lupus with congenital heart block “ANA negative” lupus Subacute cutaneous lupus erythematosus (SCLE) C2 deficiency and lupus-like syndrome DR3 gene association Subacute cutaneous lupus (SCLE) – Anti-Ro antibody-mediated SLE – The Use of Positive ANAs A positive ANA alone is not enough to diagnose SLE! Are there other autoantibodies present, e.g., anti-DNA, antiSm, anti-Ro? What are the patient’s clinical features that suggest lupus? Photosensitivity, serositis, thrombocytopenia, proteinuria, skin rashes? An ANA should only be ordered if the clinical picture warrants it! About 6-10% of people in the general population are ANA (+) Anti-Phospholipid Antibody Syndrome (APS) – Clinical and Laboratory Features Recurrent arterial and/or venous thrombosis (thrombophilia) Recurrent fetal loss (usually late miscarriages) Thrombocytopenia, autoimmune hemolytic anemia (AHA) Livedo reticularis But also: heart valve vegetations, chorea, transverse myelitis, multiple sclerosis-like syndrome, cognitive dysfunction, AVN Labs: positive antiphospholipid (APL) Abs, and/or (+) lupus anticoagulant (LAC), and/or (+) anti-2-glycoprotein 1 (anti2GPI) antibodies There is no consensus yet as to what clinical and lab features should be included or excluded in the definition of APS! Primary and Secondary APS APS can exist by itself = Primary APS (PAPS) or SLE and other connective tissue diseases can associate with APS = Secondary APS Are SLE and APS perhaps different clinical expressions in the same autoimmune spectrum? Are they one and the same? SLE and APS – Risk of Thrombosis About 20% of lupus pts have ACL and/or anti-2-glycoprotein 1 antibodies, and yet don’t have clinical thrombosis, i.e., they are at risk. However, if any of the following factors present, alone or in combination: Smoking Drug use, e.g., cocaine, and/or Estrogens, e.g., OC or HRT Perhaps hyperhomocysteinemia and other factors Clinical Thrombosis! (DVTs, MIs, CVAs, PVDs) APS – Lab Diagnostic Criteria Serologic: anticardiolipin antibodies IgG, IgM (rarely IgA), or anti- β2 glycoprotein 1 IgG or IgM antibody, by ELISA, on 2 or more occasions, at least 12 weeks apart -Test doable even if patient on anticoagulant! Functional: “the lupus anticoagulant” or LAC: Prolonged PTT, Russell viper venom test (RVVT), Kaolin clotting time, platelet inhibitor assays, etc. - Can’t do LAC if patient on anti-coagulant! False-positive RPR may be a clue that APS is present although not sensitive APS – Mechanisms of Thrombosis by APL Antibodies Endothelial cell activation (upregulating tissue factor and adhesion molecules) Platelet activation and aggregation Complement activation Macrophages Inhibitory effects on the fibrinolytic and other pathways in the coagulation cascade Targets of Anti-Phospholipid Antibodies 2-glycoprotein 1 Protein S Protein C Thrombomodulin Annexin V Prothrombin APS Abs (anti-β2GP1) also likely contribute to endothelial dysfunction and accelerated atherosclerosis in lupus – they also cross-react with oxidixed LDL Causes of Cardiovascular Complications in Lupus Procoagulant State Premature or Accelerated Atherosclerosis (multifactorial, APS) Strokes PVD MIs SLE: Therapeutic Approaches NSAIDS: but be careful with ibuprofen-other NSAIDS and aseptic meningitis Corticosteroids, including IV “pulse” Rx Hydroxychloroquine (Plaquenil®): controls and prevents SLE, anticoagulant, cardioprotective Cytotoxics: cyclophosphamide (Cytoxan®), MTX, mycophenolate mophetil (CellCept®), azathioprine (Imuran®) IVIG: short-lived correction of thrombocytopenia* Plasmapheresis: not well documented. Used for CAPS Experimental: LJP394 (B cell tolerogen for anti-DNA Abs), CTLA4Ig (abatacept), anti-C5 (? efficacy), anti-T and B cell targets (CD40-CD40L, rituximab (Rituxan®), anti-BLYS Rx (lymphostat-B, belimumab), MEDI545, an anti-IFN monoclonal antibody (MedImmune, Inc.), kinase inhibitors, prolactin inhibitors, etc Experimental combination Rx: Cytoxan® + CTLA4Ig, other combos, etc Bone marrow approaches: ablative therapy and stem cell transplant *Gonzalez EB, Truslow W, Miller SB. Intravenous immunoglobulin (IVIG) offers short-term limited benefit in lupus thrombocytopenia. Arthritis & Rheumatism 36: S228, 1993 Hydroxychloroquine (Plaquenil®) has beneficial effects in lupus and RA because: It is cardioprotective and prophylactic of cardiovascular complications It is an anti-platelet agent It prevents lupus flare-ups and progression of disease It lowers glycemia and lipids (although modestly) It downregulates the inflammatory state at different levels (DNA Abs, prostaglandins, T cell activation, etc) It is anti-malarial and anti-bacterial Espinola R, Pierangeli S, Gharavi A, Harris N. Thromb and Haemost 2002; Petri et al. Am J Med 1994; 96: 254-9 FIN Questions?