Efficacy and Safety of Vildagliptin in NODAT – a
advertisement

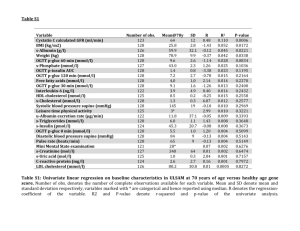
Efficacy and Safety of Vildagliptin in NODAT – a randomized, doubleblind, placebo-controlled trial Haidinger et al AJT 2014; 14: 115-123 Presented by Dr Sourabh Chand QEHB ST6/Clinical Academic Fellow Introduction • Post-transplant hyperglycaemia = morbidity, mortality – IFG – IGT • Ischaemic heart disease HR 1.39 – NODAT • premature graft failure – at 12yrs, 48% vs 70% without NODAT • Ischaemic heart disease RR 3.21 • Rx with T2DM strategies traditionally • NODAT – insulin sensitivity vs secretion 12% >10.9 GFR<30 LFTS Stable 3 months renal function OGTT – 0 & 3 months 1°outcome: OGTT – 0 & 3 months 1.1mmol/l (20) difference 2°outcomes: STOP DRUG 1 month, then OGTT 4 months – FPG, HbA1c, fasting insulin, rate S/E, ∆GFR, ACR, ∆LFTs & CNI levels Primary outcome -0.91mmol/l -0.18mmol/l -4mmol/l -0.3mmol/l • OGTT 4 months no difference from baseline • Lifestyle at 4 months (placebo) -2.2 mmol/l (±6) • HbA1c significantly different at 4 months (unsurprisingly) – “robust improvement” Insgenic Index - insulin secretion as a marker of β cell function Suppl. Table S2: calculated metabolic parameters Baseline Vildaglitpin OGIS (mL min-1m-2) 3 months 4 months p-value p-value Baseline-to-3 mo Baseline-to-4 mo 292.3 ±18.6 (N=12) 335.4 ±18.90 (N=13) 273.3 ±15.80 (N=14) 0.118 0.308 Quicki 0.444 ±0.00 (N=14) 0.464 ±0.02 (N=15) 0.437 ±0.02 (N=15) 0.525 0.674 ISIcomp 23.6 ±4.72 (N=12) 23.5 ±5.15 (N=13) 18.6 ±3.76 (N=14) 0.988 0.829 AUCg 0-120 (g/dL 2h) 26.7 ±1.44 (N=12) 21.9 ±1.49 (N=13) 26.8 ±1.47 (N=14) 0.031 0.923 AUCi 0-120 (g/dL 2h) 3.4 ±1.02 (N=12) 5.2 ±1.35 (N=13) 5.1 ±1.57 (N=14) 0.313 0.436 AUCcp 0-120 (g/dL 2h) 1.0 ±0.11 (N=11) 1.1 ±0.13 (N=13) 1.1 ±0.12 (N=14) 0.591 0.195 Insgenic Indx/deltAUCi tot (pmoL INS/mmoL G) 23.1 ±5.73 (N=12) 69.3 ±20.96 (N=13) 44.5 ±15.06 (N=14) 0.052 0.321 Insgenic Indx/deltAUCcp tot (pmoL CP/mmoL G) 0.31 ±0.05 (N=11) 0.62 ±0.13 (N=13) 0.36 ±0.06 (N=14) 0.047 0.950 Hepatic Extraction (%) 72.9 ±5.65 (N=11) 63.5 ±6.38 (N=13) 68.8 ±4.46 (N=13) 0.288 0.252 OGIS (mL min-1m-2) 285.4 ±16.00 (N=10) 310.0 ±18.10 (N=12) 293.9 ±19.90 (N=8) 0.331 0.727 Quicki 0.427 ±0.02 (N=12) 0.410 ±0.02 (N=16) 0.411 ±0.02 (N=13) 0.527 0.918 ISIcomp 13.7 ±2.8 (N=10) 11.2 ±1.44 (N=12) 11.9 ±3.42 (N=6) 0.422 0.208 AUCg 0-120 (g/dL 2h) 27.4 ±1.38 (N=10) 24.8 ±0.99 (N=12) 26.1 ±1.68 (N=8) 0.129 0.549 AUCi 0-120 (g/dL 2h) 4.2 ±0.74 (N=10) 4.8 ±0.70 (N=12) 5.0 ±0.83 (N=6) 0.560 0.297 AUCcp 0-120 (g/dL 2h) 1.1 ±0.13 (N=10) 1.3 ±0.14 (N=12) 2.1 ±0.08 (N=6) 0.365 0.256 Insgenic Indx/deltAUCi tot (pmoL INS/mmoL G) 33.0 ±6.22 (N=10) 39.9 ±8.84 (N=12) 50.6 ±11.98 (N=5) 0.548 0.541 Insgenic Indx/deltAUCcp tot (pmoL CP/mmoL G) 0.34 ±0.05 (N=10) 0.35 ±0.06 (N=12) 0.46 ±0.09 (N=5) 0.813 0.713 Hepatic Extraction (%) 65.2 ±3.63 (N=10) 64.8 ±2.72 (N=12) 59.2 ± 4.93 (N=6) 0.920 0.285 Placebo • Will changes in lipid profile affect CV outcomes (esp metabolic syndrome) Conclusions • DPP-4 inhibitors stabilise incretin hormone GLP-1 • Reduction in postprandial hyperglycaemia – Evidenced by 2hr OGTT results • No increase in BMI, relatively safe profile • Maybe more importantly in NODAT β cell protective effect • No effect on short-term effects on insulin sensitivity Remaining questions • Long term effects – Especially on CV outcome – Lipid profile – Compare other hypoglycaemics (eg metformin (eGFR)) • Is this a particular NODAT or metabolic syndrome profile? – 5 yrs post transplant, genetics, pancreatic decompensation – Deceased vs live donor – IFG/IGT patients • Other parameters – HLA mismatch, rejection episodes, multivariate analysis (∆weight, diagnoses, Bp)