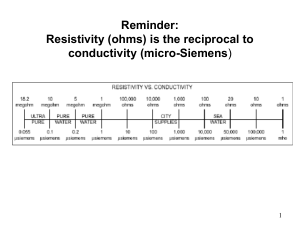
A seminar On Regulatory Aspects For Cosmetics
advertisement
A seminar On Validation Of Ampoule Filling & Sealing Machine Department Of Quality Assurance Maliba Pharmacy College 10 April 2015 1 Introduction • Validation may be defined as, “The documented act of demonstrating that any procedure, process, and activity will consistently lead to the expected results which includes the qualification of systems and equipment” 10 April 2015 2 Continued…. • The equipment validation process generally covers following steps.. – – – – – User Requirement Specification (URS) Preparation of Design Qualification (DQ) Installation Qualification (IQ) Operational Qualification (OQ) Performance Qualification (PQ) 10 April 2015 3 Stages of Qualification 4 • The previous figure depicts the most commonly used approach to the qualification process as used in the pharmaceutical industry • It shows a pyramid, which is the best way in which to plan a qualification/validation project • Investing more time in the first phases will save time and money in later and critical phases 10 April 2015 5 Who should do Equipment Validation? • The vendor or the user ? — User has the ultimate responsibility for the accuracy of the analysis results and also for equipment qualification — DQ should always be done by the user — While IQ for a small and low cost instrument is usually done by the user, IQ for large, complex and high cost instruments should be done by the vendor — OQ can be done by either the user or the vendor — PQ should always be done by the user because it is very application specific, and the vendor may not be familiar with these — PQ should be done on a daily basis 10 April 2015 6 Validation of Ampoule Filling & Sealing Machine 10 April 2015 7 10 April 2015 8 10 April 2015 9 Validation Protocol • The entire process of equipment validation is designed in the form of certain documented formats or protocols • This helps in systematizing the study of equipment validation • A validation protocol prepared by engineer or validation specialist • The protocol sections contain required procedures and forms 10 April 2015 10 Continued…. • The procedure describe “how” the system is to be validated, while the forms document these procedure and provide a written record of the performed qualification and validation processes • Each protocol package is divided into three section: – Installation qualification (IQ) – Operational Qualification (OQ) – Performance Qualification (PQ) 10 April 2015 11 User Requirement Specification • The user of the equipment has certain requirement about the equipment which he wants to use • Some of the general requirements may be stated in the form of certain parameters like… – Size of equipment – Speed of equipment – Availability of spares, change part, immediate service at reasonable cost 10 April 2015 12 Continued…. – – – – Low sound generation Lesser breakdowns Materials of construction Auto control system • This requirements are generally discussed with the suppliers and based on this discussion the selection of the equipment is done 10 April 2015 13 Continued…. For example, • In ampoule filling machine, a delivery tube provided for repetitively forcing a measured volume of liquid through the orifice of delivery tube designed to enter the constricted opening of container • The size of delivery tube depend on .. – the opening of container – the viscosity & density of liquid – speed of delivery desired 10 April 2015 14 Preparation of D.Q. and its Certification • If we are going to purchase an standard equipment then the preparation of D.Q. dose not become very important because we are accepting the manufacturer's design as it is • However, if a particular equipment is to be fabricated as per our requirements then the detailed D.Q. document become very important and essential 10 April 2015 15 Continued…. • It is advisable to work out the detailed equipment specifications by sitting together with the equipment manufacturer • It may also be advisable to perform Factory Acceptance Test (FAT) at the manufacturer’s premises before dispatch of the equipment to the purchaser 10 April 2015 16 Installation Qualification • “Installation qualification establishes that the instrument is received as designed and specified, that it is properly installed in the selected environment, and that this environment is suitable for the operation and use of the instrument” 10 April 2015 17 Continued…. • The qualification involves, – – – – – – Verification of approved purchase order Verification of invoice Check manufacturer and supplier Verification of model number and serial number Checking for any physical damage Confirm location and installation requirements as per recommendation of manufacturers – Verify that the utilities required are available – Installation shall be conducted as per instructions provided in the manual 10 April 2015 18 Continued…. • Ensure all relevant documentation is received like, − − − − User manual Maintenance manual List of change parts Electrical drawings 10 April 2015 19 Operational Qualification • O.Q. is verification of performance of the system without load • O.Q. section details the tests to be performed on the equipment to document that it operates correctly • O.Q. involve, – Verification of alarm control – Perform calibration requirements identified in the manual or established by the validation team – Operate the equipment at low, medium, and high speed as per operations manual to verify the operating control – Verify that all switches and push buttons are functioning properly – Establish procedures (SOP) for operation, maintenance, and calibration – Establish training program for relevant staff 10 April 2015 20 Performance Qualification • P.Q. is verification of performance of system with load • Filling studies will be run on all containers and fill levels • The containers will be filled in triplicate runs • If a placebo is used, it should have similar physical characteristics (viscosity, density, foaming) to the actual fill materials • The study should be run at minimum, maximum and intermittent speeds (in terms of ampoules/minute) • The filler must handle the containers without damage and without jams • Accuracy and precision must meet specifications 10 April 2015 21 • P.Q. of ampoule filling and sealing machine involve, a) b) c) d) e) 10 April 2015 Wight variation test Filling volume accuracy Particle Contamination Leaker test Oxygen content 22 a)Weight variation test : – In the absence of specific criteria, weight variation must conform to USP – In summary, all 10 units must be within 85.0 to 115.0% of target content, with a %RSD ≤ 6.0%, or not more than 1 of the 30 units outside of the 85.0 to 115.0% and no units outside of 75.0 to 125.0%, with a %RSD ≤ 7.8% 10 April 2015 23 Continued….. b)Filling Volume Accuracy: The filling accuracy should be within ±% of the adjusted and desired filling volume in accordance with the machine specification. eg. – Attention limit: ±1% – Action limit: ±2% 10 April 2015 24 Continued…. c) Particle Contamination of Ampoules during Filling & Sealing Procedure: – Ampoules should be filled with water for injection and afterward be inspected on the contamination with particles (particle classes: ≤10 μm and ≤25 μm). – The inspection can be performed with a particle counter. 10 April 2015 25 Continued…. d)Leaker test: – Should capillary pore or tiny cracks be present, micro-organisms or other contaminants may enter the ampoule, or the content may leak outside. – Leaker usually detected by submerged ampoule in a deeply colored dye solution (usually 0.5 to 1.0% methylene blue). – Limitation is that capillaries of about less than 15 um not detected by this method. 10 April 2015 26 Continued…. e) Oxygen content, – If the filler produces a nitrogen purge, the headspace gas should be analyzed for oxygen content 10 April 2015 27 Requalification • Requalification of systems and equipment should be done in accordance with a defined schedule • The frequency of requalification may be determined on the basis of factors such as the analysis of results relating to calibration, verification and maintenance • There should be requalification after changes • The extent of requalification after the change should be justified based on a risk-assessment of the change 10 April 2015 28 References... 1) R. A. Nash & A. H. Wachter, “Pharmaceutical process validation”; Third edition 2) Leon Lachman, H. A. Lieberman & J. L. Kanig, “The Theory and Practice of Industrial Pharmacy”; Third edition, Pg. No. - 667 to 674 3) Manohar A. Potdar, “Pharmaceutical Quality Assurance”, Pg. No.- 8.13, 8.14 4) Syed Imtiaz Haider, “Validation Standard Operating Procedure”, Pg. No. - 304 to 307 5) Syed Imtiaz Haider, “Pharmaceutical Master Validation Plan”, Pg. No. – 140 6) http://www.validationworld.com/ 10 April 2015 29 10 April 2015 30