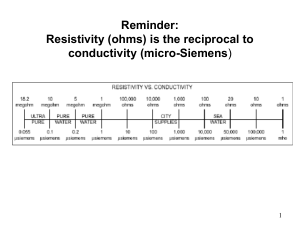
File
advertisement

VALIDATION METHODOLOGY VALIDATION : Validation is defined as the establishment of documented evidence which provides a high degree of assurance that, a planned process will consistently perform according to the intended specified outcomes. Once the system or process has been validated, it is expected that it remains in control, provided no changes are made. Incase of any modifications, or problems or equipment replacement/ relocation, Re – Validation is performed. VALIDATION ACTIVITIES Validation Activities Mainly consists of : a) b) c) d) Utilities & Equipment Qualification Cleaning Validations Analytical Instrument & Method Validations Process Validations The Utility/Equipment Qualification consists of Installation Qualification (IQ), Operational Qualification (OQ) & Performance Qualification (PQ). Equipment, Utility Qualification • Some equipment, whose correct operation itself is a measure of its performance and which is calibrated at regular intervals, may need only Installation Qualification (IQ) & Operational Qualification (OQ). Example : pH meter • IQ, OQ & PQ on major equipment, systems and Utilities must be completed and approved prior to the use of the same for manufacturing purpose. • Protocols shall be prepared, reviewed & approved for IQ, OQ &PQ studies. INSTALLATION QUALIFICATION (IQ) Installation Qualification is the documented evidence that the installed equipment conforms to the purchase specifications, manufacturer’s literature. The IQ should list and document all the Identification information of all major components, their locations, utility requirements, connections and any safety features of the equipment. IQ is intended to verify that the installed equipment matches with purchase specifications, drawings, manuals, spare parts list, supplier address and contact numbers etc. OPERATIONAL QUALIFICATION (OQ) Operational Qualification is the documented evidence that all the components of a system or piece of equipment operate according to the specifications. The OQ should provide a listing of SOPs for Operation, Maintenance and Calibration. Before initiation of OQ of an Equipment or Utility, its IQ must be completed. PERFORMANCE QUALIFICATION (PQ) • Performance Qualification is the documented evidence that a system/ piece of equipment can consistently perform and meet the required specifications under routine operation and, where appropriate, under worst-case situations. • The PQ document should include a description of the preliminary procedures required, the detailed performance tests to be performed, and the acceptance criteria for each test. PROCESS VALIDATION • Process Validation is the documentary evidence that processes are capable of repeatedly and reliably producing a Finished Product of the required Quality. • Process Validation studies examine a process under normal operating conditions to prove that the process is in control. Once the process has been validated, it is expected that it remains in control, provided no changes are made. Types of Process Validation Process validation consists of either of • Prospective Validation • Concurrent Validation • Retrospective Validation Prospective Validation : The execution and documentation of pre – approved test protocol, which is designed to prove that a process performs as intended, prior to the release of a manufactured product for distribution. CONCURRENT VALIDATION • Concurrent Validation is based on data collected during actual performance of a process already implemented in the manufacturing facility. Validation data are collected during several runs of the on-going process and evaluated to determine if the process is valid. A Protocol should be written to define the information to be collected and evaluated. RETROSPECTIVE VALIDATION • Retrospective Validation is performed whenever a product has been in production since a long time, and has not been validated either prospectively (or) concurrently. A minimum of 10 to 12 Consecutive batches will be evaluated for Consistency of Test results, Quality & Yield against Acceptance Criteria. ANALYTICAL INSTRUMENT VALIDATION The Validation of Analytical Instruments consists of Installation Qualification (IQ), Operational Qualification (OQ) & Performance Qualification (PQ). • Some Instruments, whose correct operation itself is a measure of its performance, may need only Installation Qualification (IQ) & Operational Qualification (OQ). Example : pH meter, Incubator, Freezer • IQ, OQ & PQ of the Analytical instruments must be completed and approved prior to the use of the same for other validation activities/ product testing purpose. • Protocols shall be prepared, reviewed & approved for IQ, OQ &PQ studies. ANALYTICAL METHOD VALIDATION • Analytical Method Validation is to be conducted for all identification, quantitative analytical procedures such as product strength/assay, impurity and degradation analysis etc. Validation of Analytical Method is the process of establishing any four or more of : Accuracy, Precision, Linearity, Range, Limit of Detection, Limit of Quantitation, Specificity and Ruggedness as appropriate to the type of Assay/Test. The results of Validation must be documented in an Approved Method Validation Protocol. CLEANING METHOD VALIDATION • Cleaning validation program is to be conducted for each product/ for each grouping of products to ensure that the cleaning procedures effectively remove residual products and cleaning agents from the equipment that is in contact with the product (the cleaned equipment contains an acceptable limit of previous product only). SWAB Samples / RINSE Samples are obtained & analyzed in order to prove that the cleaned equipment contains an acceptable limit of previous product only). The method of sample drawing, no. of samples to be drawn, location &equipment for sampling etc. must be documented in an Approved Method Validation Protocol.