Compliance
advertisement

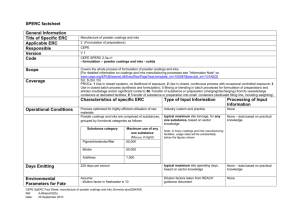
GMP 2023/2006 and Compliance Joanna Griffiths Technical Packaging Manager BRC Global Standards EU Legislation • Framework legislation on food contact materials 1935/2006 • Good Manufacturing Practice 2023/2006 • Material specific legislation • Regional legislation Overview of EC Packaging Legislation Overview of EU Food Contact Materials Legislation Overview of EU Food Contact Materials Legislation Scope of 1935/2004 • The Framework Regulation – Commission Regulation (EC) No. 1935/2004 on Materials and Articles Intended to come into Contact with Food (as amended) • Applies to materials and articles which are: – Intended to be brought into contact with food – Already in contact with food and were intended for that purpose – Reasonably expected to be in contact with food under foreseeable conditions of use Requirements • Food contact materials should be manufactured in such a way that they do not transfer constituents to food in quantities which would: – Endanger human health – Bring about an unacceptable change in the composition of food – Bring about a deterioration in the organoleptic characteristics of food Other Provisions: Traceability • Mirrors traceability requirements for food producers, materials and articles – Treated as an ingredient • Traceability at all stages • ‘One forward, one back’ principle Demonstrating Compliance Traceability at all stages of manufacture • Traceability • Process Control • Packaging Print Control Other Provisions: Compliance Declarations • Compliance declarations for relevant materials – Written declaration to demonstrate compliance with specific legal requirements – Available on demand Demonstrating Compliance Compliance • Declaration of Compliance • Senior Management Commitment • Specification and Document Control GMP… 2023/2006 • Focus is quality assurance – Not jeopardising food safety or adversely affecting food • Specific rules on inks and coatings – Currently relevant in EU Requirements • Not specific, so a system should be in place to demonstrate good manufacturing practice – Quality assurance system – Quality control system – Documentation Demonstrating Compliance Quality Assurance System “total sum of… arrangements made with the purpose of ensuring materials and articles are of the quality required to ensure conformity with the rules applicable to them.” Quality Management Policy Demonstrating Compliance Quality Control System “…ensure compliance of starting materials and intermediate and finished materials and articles with the specification determined…” • Process Control, • Hazard and Risk Analysis Demonstrating Compliance Documentation “…appropriate documentation… with respect to specifications… and processing which are relevant to compliance and safety of the finished material.” Product Safety and Quality Manual Declaration of Compliance Inks & Coatings • Relevant due to Mineral Hydrocarbon migration issues recent in EU • Guideline on GMP for inks/coatings – Formulated/applied to prevent set-off – Handled to prevent set-off – Printed surfaces not in direct contact with food Inks & Coatings Detailed Rules • Eliminate set-off in formulation and handling • No direct contact with food Process Control Controls can be set in place to ensure minimisation of risk of contamination Summary • Compliance with GMP requirements depends on knowledge of industry • Compliance with the BRC/IOP Global Standard for Packaging and Packaging Materials can aid and demonstrate compliance • Legislation designed to minimise risk of current and future food safety issues arising from packaging Thank you Hvala vam što ste