Ecosystems and Cycles
advertisement

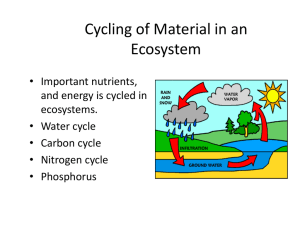
Ecosystems Important Terms • Ecosystem = a group of organisms and how they interact with their living and nonliving environment • Niche = an organisms role • Biotic = living or having lived recently • Abiotic = nonliving, long dead • Producer = converts energy from the sun, also called an AUTOTROPH • Consumer = eats other living things, also known as a HETEROTROPH – Primary, Secondary, Tertiary, Quaternary • Body of water = lake, river, pond, etc • Food chain = each organism feeds on the one below it • Food web = overlapping food chains in a community • Types of heterotrophs – Herbivores = eat plants – Carnivores = eat animals – Omnivores = eat both plants and animals (omni = all) – Detritivores = eat dead matter (called “detritus”) – Decomposers = break down organic matter • Decomposer – Organic matter = was or is living, made mainly out of carbon – Inorganic matter = non living, not made out of carbon • Biodiversity = diversity of life • Biosphere = the part of the earth where life exists • Environment = all the external factors influencing the life of organisms, such as light or food supply • Photosynthesis = to convert energy from the sun (more details to come later) • Population = the number of individuals in a species in one ecosystem • For the following slides, write in your notes whether the picture is a food web or food chain • List the producers and consumers in the pictures and what they eat. Biogeochemical Cycles • What is a Biogeochemical Cycle? – Only so much matter on earth because it is acts as a closed system. • Energy enters as sunlight, but no matter usually exits or enters. • Open system: Energy AND matter are exchanged. – These cycles act as a way to recycle matter within the biosphere from one form to another. Energy Vs. Matter • Energy is TRANSFERRED – One-way flow of energy through food-chains and food webs. • Energy from sun goes to plants, which then goes to consumers. – Each trophic level loses ~90% of energy as heat. – Only 10% of energy is used for life processes. • Matter is TRANSFORMED – This is why we have biogeochemical cycles. – Only have a given amount of matter because Earth is a closed ecosystem. Nutrient Cycles • Carbon - key ingredient in living tissue – “Carbon-based” life forms • Nitrogen - required for amino acids used in protein synthesis – What are our sources of protein? – What do we use proteins for? • Phosphorus - required for DNA and RNA – Why is this important? Water Cycle • Water is required by all living things on Earth, including us. • Cycles through atmosphere, ocean, and land Water Cycle • Major processes that bring water into the atmosphere – Evapotranspiration • Evaporation - water heats up, forming water vapor, which then moves into atmosphere. • Transpiration - water from plant leaves evaporates. – Condensation • Cloud formation as water vapor in atmosphere cools, condensing into the small droplets that form clouds. Water Cycle • Major process that brings water out of the atmosphere: – Precipitation • Droplets that formed clouds become to large and are released as snow, sleet, hail, or rain. Water Cycle • Processes on land: – Runoff • Precipitation “runs” along land until it reaches a body of water, such as a lake, river, or ocean. – Seepage (aka infiltration) • Precipitation “seeps” (moves into) soil to form ground water below the soil’s surface. – Root uptake • Plants absorb ground water from soil via their roots. Water Facts • 390,000 cubic kilometers of water evaporates and enters atmosphere each year. – Equivalent to 185,000,000,000,000,000 bottles of 2 litre soda pop. • Most evaporates from and precipitates back into the oceans… Why is this? – Ocean makes up nearly 75% of Earth’s surface. – Water that precipitates on land runs back through streams and rivers. Carbon Cycle • How is carbon taken up and released? – Photosynthesis, respiration, decomposition – Erosion, volcanic activity, and other geological activity – Fossil fuel formation (deposition) – Human activity • All these activities transfer carbon dioxide. Carbon Facts • 71% of world’s carbon is in the oceans. – Mostly as carbonate and bicarbonate (dissolved ionic forms of carbon dioxide). • 22% exists as fossils. • 3% contained in dead organic matter and phytoplankton. • 3% held in terrestrial ecosystems. • Only 1% within the atmosphere as carbon dioxide. Carbon Cycle • How can carbon get into the ocean? – Respiration by ocean animals – Precipitation that contains dissolved carbon dioxide – Erosion of carbonate rocks formed from animal skeletons and shells Phosphorus Cycle • Where is a majority of phosphorus located? – On land in rock and soil minerals. – In the ocean as sediment. – Small amount in living organisms, bound within organic molecules such as DNA and RNA as well as in skeletons of animals. – Unlike other nutrients, it DOES NOT enter the atmosphere. Phosphorus Cycle • What is the major form that phosphorus is found in? – Phosphate compounds (PO43-) Nitrogen Cycle • What form does most nitrogen exist in? – Nitrogen gas in the atmosphere (N2) • Why is this a bad thing? – This form is not readily usable by most organisms and is often considered inert. – “Inert” because of a triple covalent bond, which is a very strong chemical bond. Nitrogen Cycle • How do we get to a usable form? – – – – Bacterial nitrogen fixation Atmospheric nitrogen fixation Decomposition and excretion Haber-Bosch process = synthetic fertilizer • What are these usable forms? – Ammonia (NH3), Nitrate (NO3-) and nitrite (NO2-). Nitrogen Cycle • What can “fix” nitrogen to a usable form? – Bacteria • On root nodules of legumes such as beans; convert nitrogen gas to ammonia. • In soils, convert ammonia to nitrates and nitrites – Enzyme necessary for this requires that no oxygen be present. Nitrogen Cycle • What process removes usable nitrogen? – Denitrification • Bacteria convert nitrates back into nitrogen gas How Nutrients Effect an Ecosystem • Nutrient limitation – Similar to when a person has a deficiency in a vitamin or necessary nutritional component (like iron or calcium), ecosystems can have a deficiency in a given nutrient. – This nutrient is called the limiting nutrient, because it limits the primary productivity of an ecosystem. How Nutrients Effect an Ecosystem • What is primary productivity? – The rate at which organic material is created by producers, such as plants on land or phytoplankton in the ocean. • What happens when a limiting nutrient no longer becomes limiting? – In the ocean, this creates an algal bloom. How Nutrients Effect an Ecosystem Limiting Nutrients • A limiting nutrient limits the amount of primary productivity an ecosystem is capable of… – In the ocean, nitrogen is limiting. – In freshwater, phosphorus is limiting. • An increase in a limiting nutrient can lead to algal blooms… Algal Blooms • Increase in algae as a result of increased nutrient. – Step 1: Algae grow and reproduce rapidly. – Step 2: Algae die. – Step 3: Decomposers (bacteria) in the water take up all the oxygen via respiration as they break down the dead algae. – Step 4: Limited to no oxygen left for other animals in the water column. – Step 5: Other animals such as fish, die due to lack of oxygen. Harmful Algal Blooms • Chemicals released from bloom can be dangerous – Paralytic shellfish poisoning (picture on right - Alexandrium tamarense) – Ciguatera (picture on left - Gambierdiscus toxicus) – Neurotoxic shellfish poisoning (Gymnodinium breve)