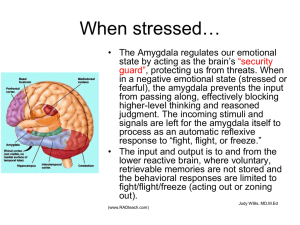
Figures from Chapter 4
advertisement

Figure 4.1 (A) Amygdala hyperactivity is present in MDD, in both depressed and remitted individuals. (B) This hyperactivity distinguishes an MDE and predicts the severity of symptoms Figure 4.2 In MDD, there is (A) hyperactivity of the amygdala (1), dACC (2), and anterior insula (3), as well as (B) in the pulvinar nucleus of the thalamus (1, 2), which relays visual information Figure 4.3 Increased severity of depressive symptoms in MDD is related to weaker functional connectivity between the amygdala and the mPFC Figure 4.4 The risk of developing MDD in response to stress is greater in individuals with higher levels of negative affect and anxiety, as indexed by the trait of neuroticism Figure 4.5 (A) Treatment of MDD with SSRIs appears to increase functional connectivity between the amygdala and mPFC and decrease amygdala hyperactivity. (B) CBT treatment of MDD resulted in decreased amygdala hyperactivity Figure 4.5 (A) Treatment of MDD with SSRIs appears to increase functional connectivity between the amygdala and mPFC and decrease amygdala hyperactivity. (B) CBT treatment of MDD resulted in decreased amygdala hyperactivity (Part 1) Figure 4.5 (A) Treatment of MDD with SSRIs appears to increase functional connectivity between the amygdala and mPFC and decrease amygdala hyperactivity. (B) CBT treatment of MDD resulted in decreased amygdala hyperactivity (Part 2) Figure 4.6 (A) Amygdyla activity in BD shows state-dependent differences: hyperactivity during mania, hypoactivity during depression, and activity equivalent to healthy during euthymia. (B) In contrast, vlPFC hypoactivity in BD is state-independent and unchanging Figure 4.7 A panic attack captured on fMRI. Initial increase in insula activity was related to feelings of discomfort (A) and followed by increased amygdala activity (B) just before the patient discontinued the scan Figure 4.7 A panic attack captured on fMRI. Initial increase in insula activity was related to feelings of discomfort (A) and followed by increased amygdala activity (B) just before the patient discontinued the scan Figure 4.8 Amygdala (A) and insula (B) hyperactivity is more pronounced in social anxiety disorder and specific phobia than in PTSD Figure 4.9 (A,B) Exposure therapy significantly decreased amygdala hyperactivity in individuals with spider phobia. (C) These decreases in amygdala hyperactivity likely reflect increases in regulatory activity of the prefrontal cortex, including the vmPFC Figure 4.9 (A,B) Exposure therapy significantly decreased amygdala hyperactivity in individuals with spider phobia. (C) These decreases in amygdala hyperactivity likely reflect increases in regulatory activity of the prefrontal cortex, including the vmPFC (Part 1) Figure 4.9 (A,B) Exposure therapy significantly decreased amygdala hyperactivity in individuals with spider phobia. (C) These decreases in amygdala hyperactivity likely reflect increases in regulatory activity of the prefrontal cortex, including the vmPFC (Part 2) Figure 4.9 (A,B) Exposure therapy significantly decreased amygdala hyperactivity in individuals with spider phobia. (C) These decreases in amygdala hyperactivity likely reflect increases in regulatory activity of the prefrontal cortex, including the vmPFC (Part 3) Figure 4.10 (A) Hyperactivity of the amygdala and dorsal ACC (yellow); and hypoactivity of the vmPFC and vlPFC (blue) in PTSD. (B) Amygdala hyperactivity to negative trauma-related imagery predicts symptom severity Figure 4.11 (A) Activity of the dmPFC is associated with decreased PTSD symptom severity measured with the CAPS. (B) Activity of the HF is also associated with decreased symptoms Figure 4.12 Individuals with PTSD exhibit impaired fear extinction and associated abnormal corticolimbic circuit function Figure 4.12 Individuals with PTSD exhibit impaired fear extinction and associated abnormal corticolimbic circuit function (Part 1) Figure 4.12 Individuals with PTSD exhibit impaired fear extinction and associated abnormal corticolimbic circuit function (Part 2) Figure 4.13 Studies with military personnel reveal that amygdala hyperactivity prior to combat exposure predicts increased sensitivity to combat-related stress Figure 4.14 (A) During processing of emotional facial expressions, there is amygdala hyperactivity in GAD only, MDD only, or comorbid for both GAD and MDD. (B) The opposite pattern of hypo- and hyperactivity is observed for the vmPFC Figure 4.14 (A) During processing of emotional facial expressions, there is amygdala hyperactivity in GAD only, MDD only, or comorbid for both GAD and MDD. (B) The opposite pattern of hypo- and hyperactivity is observed for the vmPFC (Part 1) Figure 4.14 (A) During processing of emotional facial expressions, there is amygdala hyperactivity in GAD only, MDD only, or comorbid for both GAD and MDD. (B) The opposite pattern of hypo- and hyperactivity is observed for the vmPFC (Part 2) Figure 4.15 Individuals with GAD exhibit amygdala hyperactivity during the anticipation but not the actual viewing of either aversive or neutral pictures Figure 4.16 Activity in the BNST increases as healthy participants track the probability of receiving a mild electric shock in the future Figure 4.17 In men, the propensity to experience more or less anger in contexts of reasonable provocation is predicted by the magnitude of amygdala activity to angry but not fearful facial expressions Figure 4.18 Unlike healthy individuals, who focus their attention on the eyes of a face, individuals with ASD focus their attention on the mouth Figure 4.19 (A) Amygdala activity is greater in individuals with ASD when they attend to the eyes of face stimuli. (B) The magnitude of amygdala activity is positively correlated with the amount of time attended to the eyes Figure 4.20 (A) Amygdala activity to faces habituates on repeated exposure in healthy participants but not individuals with ASD. (B) Lack of amygdala habituation in ASD is associated with social deficits Figure 4.21 Individuals with MDD exhibited relative amygdala hypoactivity to happy facial expressions but hyperactivity to sad facial expressions. The opposite pattern was found in healthy participants Figure 4.22 (A) Boys with certain traits exhibit decreased amygdala activity to fearful faces. (B) Decreased connectivity between the amygdala and vmPFC and CU traits. (C) Adolescent boys with high levels of CU traits spend significantly time attending to the eyes of fearful expressions Figure 4.22 (A) Boys with certain traits exhibit decreased amygdala activity to fearful faces. (B) Decreased connectivity between the amygdala and vmPFC and CU traits. (C) Adolescent boys with high levels of CU traits spend significantly time attending to the eyes of fearful expressions (Part 1) Figure 4.22 (A) Boys with certain traits exhibit decreased amygdala activity to fearful faces. (B) Decreased connectivity between the amygdala and vmPFC and CU traits. (C) Adolescent boys with high levels of CU traits spend significantly time attending to the eyes of fearful expressions (Part 2) Figure 4.22 (A) Boys with certain traits exhibit decreased amygdala activity to fearful faces. (B) Decreased connectivity between the amygdala and vmPFC and CU traits. (C) Adolescent boys with high levels of CU traits spend significantly time attending to the eyes of fearful expressions (Part 3) Figure 4.23 (A) Psychopaths were conscious of which stimulus was paired with pain (the CS) and perceived the CS as aversive. (B) Psychopaths showed decreased activity in amygdala, mPFC, and insula, which is consistent with failure to acquire conditioned fear response Figure 4.23 (A) Psychopaths were conscious of which stimulus was paired with pain (the CS) and perceived the CS as aversive. (B) Psychopaths showed decreased activity in amygdala, mPFC, and insula, which is consistent with failure to acquire conditioned fear response (Part 1) Figure 4.23 (A) Psychopaths were conscious of which stimulus was paired with pain (the CS) and perceived the CS as aversive. (B) Psychopaths showed decreased activity in amygdala, mPFC, and insula, which is consistent with failure to acquire conditioned fear response (Part 2) Figure 4.24 (A) Amygdala hypoactivity to fearful faces is associated with greater levels of remorseless and callous exploitation of others. (B) Amygdala hyperactivity to angry faces is associated with dangerous activities and reckless behavior Figure 4.25 Individuals with Williams syndrome exhibit amygdala hyperactivity to happy facial expressions but hypoactivity to fearful facial expressions Figure 4.26 Individuals with Williams syndrome exhibit amygdala hypoactivity to social threat but hyperactivity to nonsocial threat