PI Presentations for Clinical Trial Office
advertisement

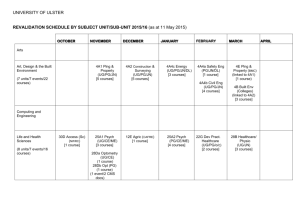
Clinical Trial Office: Support service for clinical trials performed using BMC clinical infrastructure September, 2014 Executive Summary A new clinical research support service is being launched to provide a central and comprehensive framework of financial and administrative support and oversight for clinical trials performed using BMC clinical infrastructure. The objectives of the service are to: 1. Increase the support for clinical trials through expanded institutional involvement and continued investments in the clinical research infrastructure 2. Improve cost recovery by expanding on our ability to budget and bill industry-sponsored clinical research for professional fees and hospital service costs 3. Enhance clinical research billing processes by educating the clinical research community on national best practices; and implementing policies, procedures and systems The Clinical Trial Office is a major component of this new environment and will be involved with awards for both BU and BMC. This presentation will outline why changes are necessary, what’s new (focus on the Clinical Trial Office), what has been done so far, and next steps. 2 Why is Change Necessary? The National landscape consists of tighter regulations and increased scrutiny of research costs. Noncompliance with federal regulations can lead to stringent corrective action plans and financial implications. A few examples: University of Alabama at Birmingham (2005) $3.4M • Unlawfully billed Medicare for clinical research trials that were also billed to the sponsor of research grants (double billing). Rush University Medical Center in Chicago (2005) $1.0M • Inappropriate clinical trials charges submitted to Medicare and Medicaid Beth Israel Deaconess Medical Center (2002) $ 3.2M • Improper billing of experimental cardiac devices to Medicare The following examples at BMC were identified in the recent past: Clinical Trial participants received bills in error due to incorrect registration ▪ Potential misappropriation of clinical trial expenses resulting in return of funds ▪ A Clinical Trial requiring Research MRI’s did not follow standard practice and could have resulted in research charges being charged incorrectly ▪ An independent assessment of 5 clinical trials in 2012 found that 89% of expected professional charges related to participant study visits were potentially unbilled. 3 Institutional Commitment to Clinical Research: Creating a Clinical Research Identity Being recruited to support the strategic direction of clinical research at BMC and provide clinical and scientific support to Principal Investigators and the central service functions that support clinical research Research Leadership Council Medical Director, Research Executive Director, Research Hired to bring management to the various Research Operation Functions BMC Clinical Research Being formed and will include senior faculty across BMC participating in research BMC Clinical Trial Office Research Committee Membership: A mix of BMC departmental administrators and clinical research coordinators, as well as representation from Patient Financial Services, Revenue Integrity, Investigational Pharmacy, and Radiology Membership: Chief Medical Officer (Ravin Davidoff, MD), Chief Financial Officer (Richard Silveria), Vice President, Finance (John Lindstedt), Medical Director, Research (Recruiting), Exec. Director, Research (Michael Collins) Clinical Research Admin. Task Force Supports clinical research by managing the financial, legal and administrative components of clinical trials in conjunction with Principal Investigators and Study Teams 4 A New Value Added Service for Research MANAGE | USE BU Charles River Campus Human Research Protection Program (IRB): BUMC BU Medical Campus Boston Medical Center Clinical and Translational Science Award: BUMC Intellectual Property and Commercial Ventures: CRC Note: Excludes research operations managed separately Office of Research Compliance: CRC Research Laboratory for Animal Science Center: BUMC Clinical Trial Office: BMC Effective: 2014 Investigational Pharmacy Service: BMC 5 Clinical Trial Office Support Services The Clinical Trial Office (CTO) will support clinical research by managing the financial, legal and administrative components of clinical trials in conjunction with Principal Investigators (PIs) and Study Teams. The CTO is involved in the following functions: Study Initiation Medicare Coverage Analysis (MCA) Budget Development (comprehensive negotiation support) Negotiation of Contract Terms and Conditions Financial and Billing Management Reporting and Auditing Account Reconciliation and Closeout The new program leverages best practices from peer institutions, including Partners Healthcare, Beth Israel Deaconess Medical Center, Tufts Medical Center, and Children’s Hospital. In addition to the local peer review; Jim Moran, Executive Director, Healthcare Advisory Services at Ernst & Young (formerly of Washington University) and Erika Stevens, Senior Manager (formerly of Dartmouth Hitchcock Medical Center and Columbia University Medical Center) are contributing insight to ensure that the CTO is consistent with standard practices. 6 Benefits of CTO Support Service Function Details Centralized Support One office for BMC research to support contract management, research-related and patient-related billing, and closeout Budgets Development and negotiation with sponsors for each protocol and study by a Clinical Trial Financial Analyst (CTFA) Professional Fees Partnership between CTFA and professional groups to correctly budget and bill the sponsors to more accurately reflect physician time spent on clinical trials and increase revenue recovery Research Committee Involvement of senior faculty in setting research identity Clinical Research Administrative Task Force Involvement of study coordinators and departmental administration in changes to policies, processes and practices for clinical trial management 7 PI Engagement is Important for Success! Although the CTO will strive to minimize additional administrative burdens on Principal Investigators, increased participation is needed to ensure the continued success of the institution’s clinical research program. Function Details Medicare Coverage Analysis Requirement for all clinical trials and involves PI effort Billing Grids Requirement for all clinical trials and involves study team effort Study Participant Charges Greater scrutiny to ensure patient charges are reconciled and meet Medicare billing standards PI Attestation Investigator attestation that study funding will be administered in good faith (study initiation) and charges have been properly submitted (close out). 8 CTO Recruitment Director, Clinical Trial Office: Selvin Ohene • Formerly Administrative Director of Cancer Center, Tufts Medical Center Senior Clinical Trial Financial Analyst: Dean Robinson • Formerly Clinical Trial Financial Analyst, Massachusetts General Hospital (CCPO) Senior Clinical Trial Financial Analyst: Allisson Dugan • Formerly Senior Research Administrator, Beth Israel Deaconess Medical Center Clinical Research Attorney: Meghan Garland • Formerly Senior Agreement Associate, Partners Healthcare 9 VelosCT Boston Medical Center has made a capital investment ($600K+) to implement a fully functional clinical trial management system (Velos eResearch) that will assist with: • • • • Participant recruitment and accrual Scheduling, follow-up, retention and management Financial management Reporting for all clinical trials The implementation will be staged in three main phases, involve stakeholders with various crossfunctioning subject matter expertise, and external support and is expected to be complete May, 2015 . Phase I: System setup, configuration and onboarding new trials Phase II: Data migration of selected existing studies Phase III: Additional interfaces created and integrated over next three years Executive Sponsor: Michael Collins: Executive Director, Research Project Manager: Garo Stone-DerHagopian: Associate Director, Research Business Intelligence 10 Appendix 11 Clinical Trial Finance Lifecycle CTO INVOLVEMENT PATIENT VISIT RESEARCH vs. SOC CHARGE CAPTURE BILLING & CODING CLAIMS EDITING Study Start Up PATIENT REGISTRATION INVOICING Post Award COLLECTIONS PATIENT CONSENT PreAward COVERAGE ANALYSIS Close Out POST TO CORRECT RESEARCH ACCT. RECONCILIATION IRB BUDGETS REPORTING CONTRACT MGMT IRB APPROVAL ACCOUNT CLOSEOUT 12 Policies and Procedures (Ernst & Young Engagement) Study Initiation Post-Award • A total of 14 study initiation policies and procedures developed by Executive Director, Research were reviewed by EY with minimal revision. An additional 7 new policies and procedures were created by EY to enhance BMC pre-award processes. • A total of 21 existing post-award policies and procedures were reviewed by E&Y with minimal revision. An additional 13 new policies and procedures were created by EY to enhance BMC post-award processes. E&Y Advisory Services reviewed 35 policies and procedures (some in draft). Minimal or no edits were suggested for 27 policies with proposed edits to 8 policies. E&Y drafted an additional 20 policies to support research operations. 13 Training and Educational Opportunities The CTO will work in collaboration with the Associate Director, Research Operations to provide the clinical research community with opportunities for training and education. There will be multiple channels available to PIs and their study teams to receive training: • Live group training sessions (available in Fall, 2014) • Targeted online training (via Healthstream) (TBA) • Seminar series (TBA) The following training sessions will be required for Investigators/study teams involved in clinical research: Training Session PI requirement Study team requirement Clinical Research Billing • Medicare Coverage Analysis • Budget Development • Charge Capture (SOC vs. Research vs. Blended study visits) Clinical Trial Management System (VelosCT) optional Time commitment is expected to be 1 hour per topic area. 14