The Electromagnetic Spectrum and Sources of Light
advertisement
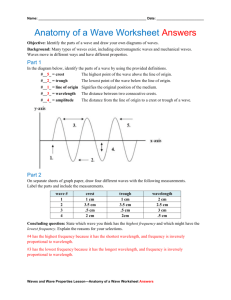
The Electromagnetic Spectrum and Sources of Light What is Light? • Light is a form of energy. It is one of several forms of energy we receive from the Sun as part of the solar radiation it produces. • The energy from the Sun travels to Earth as electromagnetic waves. These waves can pass through the vacuum of space or materials on the Earth’s surface. • Electromagnetic = it affects both electrical and magnetic fields! Energy in Waves • A wave is a disturbance that transfers energy from one point to another without transferring matter. • Waves can be described using their physical characteristics and/or properties. Properties of Waves • Crest – the highest point on a wave. • Trough – the lowest point on a wave. • Rest Position – the level of a fluid (like water) when there are no waves passing through it. Properties of Waves The Motion of the Ocean • Wavelength (λ) – The distance from one place on a wave and the next similar place on the wave. (Crest to crest or trough to trough are most common.) – Wavelength is measured in metres (m). • Amplitude – The wave height from the rest position to the crest, OR, the wave depth from the rest position to the trough. – Amplitude shows the energy carried by the wave. • Frequency (ƒ) – The rate of repetition of a wave. – Frequency is measured in Hertz (Hz) which in cycles per second. Frequency & Wavelength • Frequency and wavelength have an inverse relationship. (As one goes up, the other goes down.) • The speed (v) of the wave is determined by multiplying the frequency (ƒ) by the wavelength (λ). v=ƒxλ PROPERTIES of Waves https://www.youtube.com/watch?v=01Jcz6t9UQg The Spectrum of Visible Light • The light that comes from the Sun or a source like a candle is called visible light. It is the light that is visible to the human eye. • Over 300 years ago, Sir Isaac Newton passed visible light through a triangular piece of glass called a prism and separated the light into its component colours. • The pattern of the colours makes the name “ROY G. BIV” and is known as the spectrum of visible light. The Visible Light Spectrum The Electromagnetic Spectrum • Visible light is just one type of electromagnetic energy. There are also radio waves, microwaves, X-rays, and gamma rays. All of these are found in the solar radiation of the Sun. • All of the electromagnetic energy can be arranged to form the electromagnetic spectrum. It arranges all of the energy in solar radiation in order from weakest to strongest. • Visble light is only a small fraction of the electromagnetic spectrum. The Electromagnetic Spectrum Sources of Light Categories of Light • We categorize light based on the source of the light. • We say an object is luminous when it emits or produces light. • Light may be: – – – – – Incandescent Fluorescent LEDs Chemiluminescent Bioluminescent Incandescent Light • Incandescence is the light produced when an object is heated to a really high temperature. • Older light bulbs have a filament, that gets very hot as electricity is passed through it, that gives off the light. • These lights are inefficient and not good for the environment because most of the energy put into them is lost as heat (rather than light). How A Light Bulb Works! http://www.youtube.com/watch?v=YnMP1Uj2nz0 Fluorescent Light • Fluorescence occurs when a substance absorbs high energy UV radiation and immediately releases that energy as visible light. • A current is passed through the tube of the light. It excites the mercury and argon in side the tube and they give off UV light. • This UV light reacts with a chemical that coats the tube and it glows – the chemical coating shows fluorescence!!! How Fluorescent Lights Work! http://www.youtube.com/watch?v=rS5LC2aH0c4 LEDs • LED stands for light emitting diode. • An electric current passes through an LED and it emits light. • LEDs are very energy efficient and are replacing many of the other forms of light. • LEDs save energy, last longer and stay cooler. Chemiluminescence • Chemiluminescence occurs when two chemicals react and give off light energy. • Light sticks contain two chemicals that mix when you crack the stick and shake it to mix the chemicals. • A dye used inside the tube will determine the colour of the stick. • Party-goers, campers and law enforcement all make use of this chemical light form. Chemiluminescence Bioluminescence • Bioluminescence occurs in a living organism. Chemicals produced in the organism are mixed together to produce the light. • We see this phenomenon in fireflies, jellyfish, fungi and bacteria. Bioluminescence Bioluminescence What about the Moon? • The moon appears to give off light but it is actually non-luminous – meaning it does not produce its own light. • The moon only appears to give off light because it reflects light from a luminous object – the Sun. Light Off of the Moon THE END