Zodrow_DeGrandi_proj2_BrownianMotion_Slides
advertisement

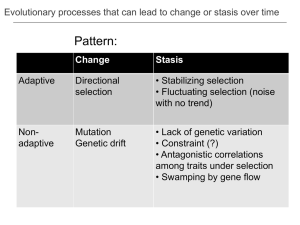
Teachable Unit: Brownian Motion Created by: Claudia De Grandi and Katherine Zodrow May 2013 claudia.degrandi@yale.edu katherine.zodrow@yale.edu • • Unit Summary This unit contains materials for 2 or 3 class periods. Parts of this unit can stand alone. Teaching Materials Include Powerpoint slides detailing unit Powerpoint slides to be used in the classroom 2 Matlab modules (for beginners) to help explain Brownian motion In class quiz/questions Homework Part 1 (75 min) 1.Introduce learning goals 2. Perform a 1D random walk as a class 3. History of Brownian motion (lecture) 4.Reflection and discussion Part 2 (75 min) 1. Present and discuss the solutions to the homework (from Part 1) 2.Computer lab group activity with Matlab (Module I) - Students follow instructions on handout to simulate and analyze 1D random walks - Students hand in a final lab report - Students are given final answer key sheet - TAs and Instructor available for in-class help Part 3 (75 min) 1.Introduction to data analysis: How do researchers use Brownian motion? 2.Computer lab group activity with Matlab on 2D random walks (Module II) 3. Final question: Estimate the radius of an atom 4.Discuss solution of the question 5.Reflection, final comments on initial learning goals. Assessment • • • • • • Initial reflection on learning goals questions (see slide 9) Initial multiple choice quiz about binomial distribution (see slide 11) Homework (end of Part 1) on diffusion in different viscosities 2 Matlab Modules, to be turned in as a short lab report In-class final problem questions about the size of an atom Final homework/report: revised and detailed answers to learning goals questions Materials needed • • • • • • coin to flip for each student a relatively spacious room to implement the random walk activity or a large white board and Post-it stickers a computer and screen to project slides Device for students to use Learning Catalytics (formative assessment questions and class random walk activity) Computer with Matlab for each student group Handout for students with a copy of the slides Classroom Slides Brownian motion, Atoms and Avogadro’s Number •How do we know atoms exist? •What is the size of an atom? •How would you observe an individual atom? Brownian motion, Atoms and Avogadro’s Number •How do we know atoms exist? •What is the size of an atom? •How would you observe an individual atom? Suggestion: make a ‘diary’ to keep track of your learning process At the end of the 3 lectures your homework will be to summarize what you have learned and give your best answers to those questions. Today in class, we will 1. Review the binomial distribution: quiz 2. Perform a 1D random walk as a class and extract our diffusion coefficient 3. Review the history of Brownian motion: - R. Brown(1827): botanist observing motion of pollen grains - Einstein’s theory and connection to Avogadro’s number (1905) - Perrin’s experiment (1908) Binomial Distribution reminder p = probability of one success probability of k successes in n trials average number of successes : variance : Learning goals: • understand how the variance depends on time •extract the diffusion coefficient D Learning goal: • understand how to extract the diffusion coefficient from 2D images • relate the diffusion coefficient to the Avogadro’s number QuickTime™ and a decompressor are needed to see this picture. 50 100 150 200 250 300 350 400 50 100 150 200 250 300 350 400 450 500 550 50 100 150 200 250 300 350 400 50 100 150 200 250 300 350 400 450 500 550 365 370 375 380 385 290 295 300 305 310 315 320 ideal gas constant Ideal gas law Pressure Temperature Volume ideal gas constant Pressure Ideal gas law Temperature Volume In our case: water # of moles mole=as many molecules as in 12 grams of 12C mole= Avogadro’s number(NA) of molecules total number of molecules Boltzmann constant ideal gas constant Pressure Ideal gas law Temperature Volume In our case: water historically # of moles Einstein’s theory of Brownian motion! mole=as many molecules as in 12 grams of 12C mole= Avogadro’s number(NA) of molecules total number of molecules Boltzmann constant Einstein’s theory Einstein’s theory Einstein’s theory Einstein’s theory Diffusion coefficient Friction coefficient Radius of green particles viscosity Einstein’s theory Diffusion coefficient measurable! in Brownian motion Friction coefficient Radius of green particles viscosity Einstein’s theory Diffusion coefficient measurable! in Brownian motion Friction coefficient time known quantities Today’s Recap Diffusion coefficient of 1D random process Diffusion coeff. of 2D brownian particles Einstein’s theory Avogadro’s number! Brownian motion, Atoms and Avogadro’s Number •How do we know atoms exist? •What is the size of an atom? •How would you observe an individual atom? Homework 1) At 20 °C, the dynamics viscosity η of water is 10-3 Pa*s. Glycerol is 1 Pa*s. We place particles in these two solutions, holding everything else constant. Give a quantitative relationship for the diffusion of these particles in these two solutions. 2) Sketch a plot that compares <x2>vs. time for particles in each of these solutions. Today in class, we will 1. Review solutions to the homework 2. Use Matlab software to simulate and analyze in details 1D random walks - Work in groups of 2/3 people Follow instruction on handout Hand in a report by the end of the lecture Today in class, we will 1.Use Matlab software to simulate and analyze 2D Brownian motion (like reproducing Perrin’s exp. images) - Work in groups as Module I, hand in final report - You will extract the Avogadro’s number from your data 2.Group problem: estimate the size of a molecule from Avogadro’s number 3.Final discussion on learning goals and final homework Estimate of molecular radius Assume Avogadro’s number NA= 6 X1023 NA is the total number of molecules in a mole Reminder Ideal gas law: Work in groups to find an estimate of the size of a molecule in a mole Estimate of molecular radius volume of a mole at room Temp. (T=300) and 1 atm.(100kPa) estimate of particles radius Brownian motion, Atoms and Avogadro’s Number •How do we know atoms exist? •What is the size of an atom? •How would you observe an individual atom? Final homework/report/reflection: write down your best answers, compare with your initial guess, discuss what are the most important things you have or have not learned during this teaching unit • • • • Additional reading Haw, M D. (2002) Colloidal suspensions, Brownian motion, molecular reality: a short history. J. Phys. Condens. Matter 14:7769. Philip Nelson’s book: Biological physics: Energy, Information, Life (Chap. 4). Random Walks in Biology, Howard Berg Investigation on the theory of The Brownian Movement, Albert Einstein, Dover Publications (1956) (original Einstein’s paper on Brownian motion).