Spectroscopy - Bryn Mawr College
advertisement

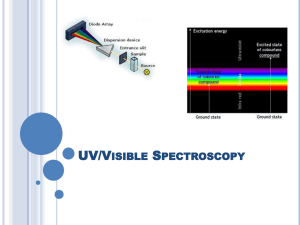
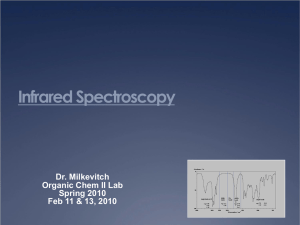
Investigating art Why?? to help direct restoration to help conservation to aid interpretation just to “know” ( see: “Is a painting ever done?” Ball, Time as Painter, Chapter 11) Methods: 1. spectroscopy (“quantify interaction with light”) 2. chemical behavior ( “does it react?”) 3. microscopy (“visual with a microscope”) lots Tickling Out the Truth Objectives for today: Objectives for Tuesday: Understand: 1. what is spectroscopy Lab 1. 2. how it quantifies color Measure visible spectra of mauveine and pseudomauveine 3. what is meant by light absorption, reflectance, transmittance 4. how to generate a reflectance (transmittance) spectrum from an absorption spectrum and vis versa. 5. how broad spectroscopy is, e.g., how infrared spectroscopy is possible, UV. little 2. Obtain Infrared spectrum of carmine and of mauve 3. Run ESI-MS on mauveine and pseudomauveine The Basics of Spectroscopy: light source sample detector the “black box” the brains for the box A= e*b*c lots little lots little Absorption Spectrum of Blue Filter lots little Transmittance Spectrum of Blue Filter Transmission Absorption light color “thrown away (observed) Reflection Absorption Transmission and Reflection effectively do the same thing: throw away unabsorbed colors lots lots Transmission Spectrum little Absorption Spectrum little Our eyes are pretty good, but not as quantitative as a spectrum from spectroscopy Example 1. Shiraz wine does look different from Chianti (!) Shiraz Chianti wavelength 550 600 650 700 750 nm the fate of an open bottle of Shiraz: Example 2. Black Swan Shiraz, fresh: 530 nm Shiraz does taste different when left open. Black Swan Shiraz, open 12 days: 510 nm Watercolor Pigment Reflectance Curves: How does ultramarine blue Reflectance curve for ultramarine blue watercolor pigment differ from phthalocyanine blue? Reflectance curve for phthalocyanine cyan http://www.handprint.com/HP/WCL/color2.html#reflectance © handprint.com, 2004, Bruce MacEvoy Watercolor Pigment Reflectance Curves: Reflectance curve for ultramarine blue watercolor How does ultramarine blue pigment differ from A yellow pigment? Reflectance curve for hansa yellow watercolor http://www.handprint.com/HP/WCL/color2.html#reflectance © handprint.com, 2004, Bruce MacEvoy “Light” is a term that refers to electromagnetic radiation. And electromagnetic radiation are waves of different energies that extend over a broad range: frequency, Hz 10 26 10 24 cosmic 10 22 gamma wavelength, nm 10 -8 10 -6 10 -4 10 20 10 18 10 16 X-rays 10 -2 1 10 14 10 12 UV Vis 10 2 10 10 IR 10 4 10 6 10 8 10 6 Radio 10 8 10 4 induction 10 2 Hz power 10 10 10 12 10 14 10 16 nm If the electromagnetic spectrum were a piano keyboard….. the visible spectral region would be just one key! light source sample detector interacts with light; “removes” (absorbs) some light components X-rays Interaction type: Observed results: UV Vis electronic none none IR vibration color heat Chromate, CrO4 2“chromophore” in chrome yellow X-rays Interaction type: UV Vis electronic Observed results: none X-ray affects inner Electrons: Higher energy none IR vibration color UV/vis affects outer electrons: Lower energy heat X-rays Interaction type: UV Vis electronic Observed results: none where in painting: penetrates all none IR vibration color surface X-rays X-rays interaction strength depends on number of electrons: Pb >> Zn, Ti Lead white scatters X-rays more than zinc white or titanium white or most other pigments (except HgS or Ba-pigments) heat below paint IR wavelength selected to interact most with black of underdrawing Infrared Spectroscopy Instrumentation An infrared spectrophotometer is composed of: 1) an IR light source, 2) a sample container, 3) a prism to separate light by wavelength, 4) a detector, and a recorder (which produces the infrared spectrum). SEE: Alizarin Carmine Indigo http://www.chemistry.ccsu.edu/glagovich/teaching/472/ir/instrumentation.html Investigating the Renaissance a Harvard site with examples of spectroscopic methods Feast of the Gods An analysis by WebExhibits begin with historical context: •when did artist/work occur •workshop/atelier context and influences? •location •information on commission/motivation for work Electrospray Ionization Mass Spectrometry ESI-MS ‘black box’ Molecules in gas phase as ions + or - magnetic field separates light and heavy mass ions mass detector Electron impact ionization Calculated mass of mauveine C24H19N4 = 363 g/mol 24 C = 24 x 12 = 288 19 H = 19 x 1 = 19 = 363 Calculated mass of pseudomauveine C26H23N4 = 391 g/mol 4N= 26 C = 26 x 12 = 312 4 x 14 =2356H = 23 x 1 = 23 4N= 4 x 14 = 56 = 391 Electrospray ionization method makes tiny droplets Vacuum removes solvent Electric potential creates + and - ions All Mo molecules are identified by a characteristic series of lines: Mo isotopes: 92Mo, 94Mo, 95Mo, 96Mo, 97Mo, 98Mo,